Abstract
Large lobar intracerebral hemorrhages (ICHs) can cause rapid neurological deterioration, and affected patients have low rates of survival and functional independence. Currently, the role of surgical intervention in the management patients with lobar ICHs is controversial. Minimally invasive technologies have been developed which may potentially decrease the operative morbidity of ICH surgery. The aim of this case report is to describe the technical aspects of the use of a novel minimally invasive endoport system, the BrainPath (NICO, Indianapolis, IN, USA), through an eyebrow incision for evacuation of a large lobar hematoma. An 84-year-old female presented with a left frontal ICH, measuring 7.5 cm in maximal diameter and 81 cm3 in volume, secondary to cerebral amyloid angiopathy. Through a left eyebrow incision, a miniature modified orbitozygomatic craniotomy was performed, which allowed endoport cannulation of the hematoma from a lateral subfrontal cortical entry point. Endoport-assisted hematoma evacuation resulted in nearly 90% volume reduction and improvement of the patient's functional status at clinical follow-up. We found that minimally invasive endoport technology can be employed in conjunction with conventional neurosurgical skull base principles to achieve safe and effective evacuation of large lobar hematomas in carefully selected patients.
The role of surgical intervention in the management of supratentorial, lobar intracerebral hemorrhage (ICH) is a subject of debate.12) The recent Surgical Trial in Lobar Intracerebral Hemorrhage (STICH II) did not find significantly better outcomes in patients who underwent early (within 48 hours of ictus) surgery compared to conservative management, but the results suggested a small degree of clinical benefit from surgical intervention.11) Large lobar hematomas can precipitate rapid neurological decline due to perihematoma edema, local mass effect, cerebral herniation, and elevated intracranial pressure (ICP). Thus, surgical ICH evacuation can, in some cases, prevent progressive neurological deterioration and facilitate clinical recovery. In order to decrease the physiological stress and operative morbidity associated with conventional techniques for ICH surgery, a number of minimally invasive approaches have been adopted, although no single modality has been widely validated or routinely used for ICH patients.13)16)17) The aim of this case report is to describe the technical nuances of a novel endoport system, the BrainPath (NICO, Indianapolis, IN, USA), for minimally invasive evacuation of a large lobar hematoma through an eyebrow incision.
The BrainPath endoport system consists of an inner obturator within an outer sheath, which is 13.5 mm in diameter and available in three different lengths (50 mm, 60 mm, and 75 mm). The outer sheath has an arm, which can be affixed to a Greenberg retractor in order to maintain the endoport's access to the target lesion. The tip of the inner obturator, which extends 15 mm beyond the outer sheath, is blunt and tapered. This allows for minimal trauma to the cortex and white matter fibers during cannulation of the endoport. An 8 mm obturator tip is available, which is used exclusively with the 50 mm outer sheath, for cannulation of shallow targets. Of note, image guidance with stereotactic neuronavigation is crucial to accurate endoport placement in an optimal trajectory and to an appropriate depth.
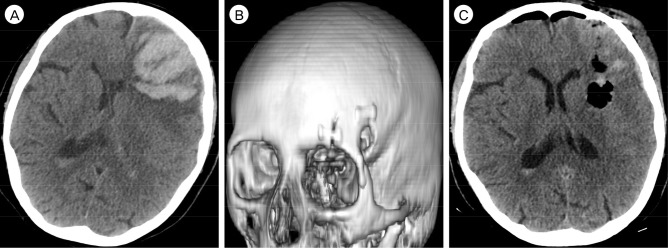
An 84 year-old female with a history of hypertension, hyperlipidemia, atrial fibrillation, and chronic kidney disease presented with a sudden onset of headache and aphasia. Upon arrival in the emergency room, her systolic blood pressure was 160 mmHg, modified Rankin Scale (mRS) score was 4, and Glasgow Coma Scale was 10. Brain computed tomography (CT) showed a large, 7.5 × 4.3 × 5.0 cm left frontal lobar ICH (volume 80.6 cm3) with intraventricular extension (ICH score 4; Fig. 1A).9) The patient was administered a platelet transfusion to counteract the effects of aspirin, which she was taking on an outpatient basis. Due to the lobar location and large size of the hematoma, as well as the associated midline shift and local mass effect, we elected to proceed with surgical evacuation, which was undertaken within 12 hours of ICH onset.
After image registration of a thin-slice brain CT (slice thickness less than 1 mm) with the StealthStation (Medtronic, Minneapolis, MN, USA) frameless stereotactic neuronavigation system, we planned a trajectory for endoport placement through the long axis of the hematoma. In order to avoid entering through the cortex of the left inferior frontal gyrus (i.e., anatomic Broca's area), we opted for an approach through a lateral subfrontal entry point, where the hematoma rose to the cortical surface. To expose the planned cortical entry point, we decided to perform a left-sided miniature modified orbitozygomatic craniotomy (mini-mOZ) through an eyebrow incision.
Following induction of general anesthesia, three-point fixation with the Mayfield Skull Clamp System (Integra, Plainsboro, NJ, USA) was applied to the patient head, and secured in cervical and capital extension with rotation toward the right. After making an incision along the superior border of the left eyebrow from the keyhole laterally to the supraorbital notch medially, the frontalis muscle and galea were divided with monopolar electrocautery, and a pericranial graft was harvested. The orbital rim was exposed from the frontozygomatic suture to the supraorbital notch, and the temporal muscle was reflected posteriorly to uncover the keyhole. A burr hole was made at the keyhole, and bony cuts were made at the frontozygomatic suture and lateral to the supraorbital notch. The fracture lines through the orbital roof were propagated posteriorly with an osteotome to release the bone flap, thereby completing the mini-mOZ craniotomy (Fig. 1B).
A small, cruciate dural opening was made in order to minimize any loss of cerebrospinal fluid, but of adequate size so as to accommodate the endoport. The hematoma was immediately visualized upon dural opening, breaching the cortical surface. Under neuronavigation from StealthStation, the BrainPath endoport system, with the 60 mm outer sheath, was used to cannulate hematoma. The endoport was advanced along the planned trajectory into the deep portion of the hematoma, after which the inner obturator was removed. We then proceeded with hematoma evacuation using the operating microscope and standard bimanual microsurgical technique. A cluster of vessels at the deep portion of the hematoma with an abnormal appearance was resected. Histopathological analysis of the specimen was consistent with cerebral amyloid angiopathy. After evacuation of the deep portion of the hematoma, the outer sheath was sequentially retracted, allowing additional removal of peripherally located portions of the hematoma which collapsed into the operating field. Upon complete removal of the outer sheath, we used the Myriad (NICO), an automated, non-heat generating soft tissue resection device, to perform further hematoma resection at the cortical surface, which was noted to be quite firm.
At the completion of the hematoma evacuation, wound closure was performed in a standard layered fashion, and the craniotomy bone flap was affixed to the skull with a titanium plating system. Postoperative brain CT showed nearly 90% reduction in ICH volume and significantly decreased midline shift and local mass effect (Fig. 1C). The patient was discharged one week after surgery to a nursing facility (mRS of 4 at discharge), and subsequently improved to a mRS of 3 at five months clinical follow-up.
Spontaneous ICH comprises 10–20% of stroke and portends a poor prognosis, with relatively high rates of mortality, functional dependence, and cognitive impairment.7) ICH volume has been consistently correlated with patient outcomes. Broderick et al. found that patients with an ICH volume greater than 60 cm3 had a 30-day mortality rate of over 80%.2) Even ICH volumes greater than 30 cm3 have been shown to result in a vanishingly low rate of functional independence.2) The patient in the present study had an ICH score of 4, which has been found to carry a 97% risk of 30-day mortality. Additionally, many ICH patients are saddled with multiple medical comorbidities, frequently requiring medical therapy with antiplatelet agents and anticoagulation, which further complicates the decision to provide surgical intervention.4)10)
Minimally invasive approaches may expand the interventional armamentarium for ICH patients by improving the safety of hematoma evacuation, particularly in medically ill patients, without significantly compromising efficacy. Although further studies are necessary to define the role of endoport-assisted microsurgery in the management of ICH patients, our preliminary experience has shown that it is an effective approach for evacuation of large lobar hematomas. Furthermore, we show that this novel endoport system can be combined with minimally invasive surgical techniques, such as a mini-mOZ craniotomy through an eyebrow incision, to access hematomas in eloquent brain regions via safe trajectories through non-eloquent cortical entry points. As with any surgical approach, careful case selection is crucial to optimizing the outcomes of this technique. We believe that the morphology of a hematoma, rather than its volume, should be heavily considered when evaluating a patient for endoport-assisted ICH surgery. Specifically, significant volume reduction is more likely to be achieved with endoport cannulation of hematomas with a distinct long-axis, such as cylindrical or wedge-shaped lesions. By allowing the peripheral portions of the hematoma to collapse inward following evacuation of the central hematoma, even very large volume hematomas greater than 60 cm3 can be successfully evacuated with this approach, which may represent an advantage of the endoport over catheter-based techniques.13)14)
In order to access the deep portion of lobar hematomas, the use of traditional retractor systems may result in an uneven distribution of force against the tissue. This can result in a predisposition toward venous congestion and ultimately infarction, cerebral edema, and seizures. In contrast, the endoport exerts a relatively equal amount of radially oriented force in all directions, thereby potentially reducing the risk of postoperative complications related to prolonged brain retraction.3)5)6)8)15) Combined with the relatively small skin incision, craniotomy, and dural opening through with the endoport can be deployed, this technology allows for both minimally invasive and minimally traumatic evacuation of large lobar hematomas. These unique advantages of endoport-assisted microsurgery over conventional approaches may improve postoperative recovery and diminish the duration of hospital stays for ICH patients.
Despite the significant potential for endoport technology to improve ICH surgical outcomes, its limitations should be delineated. Due to the reliance of targeting on neuronavigation, even small errors in image co-registration or trajectory planning can result in suboptimal endoport cannulation. Although it would be highly unlikely to completely miss a large lobar hematoma, an errant endoport trajectory can reduce the proportion of hematoma which is evacuated and, thus, potentially affect the clinical outcome. Additionally, while the outer sheath can physically impede hemorrhage from the subcortical white matter fibers and superficial cortex, another challenge of an endoport-assisted approach that may arise intraoperatively is obscuration of the operative field by bleeding from vessels at the depth of the hematoma resection cavity. Furthermore, due to the relatively confined surgical corridor of the endoport, it may be difficult to manipulate particularly firm portions of a hematoma. However, in contrast to endoscopic approaches, the hindrances of the endoport can be partially overcome by the use of the operating microscope and bayoneted instruments, which improve surgical efficiency by allowing the employment of bimanual microsurgical technique.1) Future directions include identification of optimal patient and lesion characteristics for an endoport-assisted approach, analysis of perioperative and long-term outcomes in large cohorts, and comparing the effectiveness of endoport-assisted ICH surgery to conventional and other minimally invasive techniques.
The BrainPath endoport system can be employed in conjunction with minimally invasive skull base approaches, such as a mini-mOZ craniotomy through an eyebrow incision, for successful evacuation of large lobar hematomas. The endoport allows the use of trajectories to the hematoma which avoid eloquent brain regions, thus potentially facilitating neurological recovery. Although additional studies are necessary to determine the long-term outcomes and optimal patient and hematoma characteristics for endoport-assisted surgery, our initial experience supports its safety and efficacy in appropriately selected ICH patients.
References
1. Beynon C, Schiebel P, Bosel J, Unterberg AW, Orakcioglu B. Minimally invasive endoscopic surgery for treatment of spontaneous intracerebral haematomas. Neurosurg Rev. 2015; 7. 38(3):421–428. discussion 428. PMID: 25687253.

2. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993; 7. 24(7):987–993. PMID: 8322400.

3. Chen CJ, Caruso J, Starke RM, Ding D, Buell T, Crowley RW, et al. Endoport-assisted microsurgical treatment of a ruptured periventricular aneurysm. Case Rep Neurol Med. 2016; 2016:8654262. PMID: 27195160.

4. Ding D. Surgical strategies for spontaneous basal ganglia hemorrhages. Br J Neurosurg. 2015; 6. 29(3):447. PMID: 25529967.

5. Ding D, Przybylowski CJ, Starke RM, Sterling Street R, Tyree AE, Webster Crowley R, et al. A minimally invasive anterior skull base approach for evacuation of a basal ganglia hemorrhage. J Clin Neurosci. 2015; 11. 22(11):1816–1819. PMID: 26142050.

6. Ding D, Starke RM, Webster Crowley R, Liu KC. Endoport-assisted microsurgical resection of cerebral cavernous malformations. J Clin Neurosci. 2015; 6. 22(6):1025–1029. PMID: 25769248.

7. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013; 1. 127(1):143–152. PMID: 23283859.
8. Greenfield JP, Cobb WS, Tsouris AJ, Schwartz TH. Stereotactic minimally invasive tubular retractor system for deep brain lesions. Neurosurgery. 2008; 10. 63(4 Suppl 2):334–339. discussion 339-40. PMID: 18981840.

9. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001; 4. 32(4):891–897. PMID: 11283388.
10. Liotta EM, Prabhakaran S. Warfarin-associated intracerebral hemorrhage is increasing in prevalence in the United States. J Stroke Cerebrovasc Dis. 2013; 10. 22(7):1151–1155. PMID: 23287421.

11. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; 8. 382(9890):397–408. PMID: 23726393.

12. Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010; 9. 41(9):2108–2129. PMID: 20651276.
13. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013; 3. 44(3):627–634. PMID: 23391763.

14. Newell DW, Shah MM, Wilcox R, Hansmann DR, Melnychuk E, Muschelli J, et al. Minimally invasive evacuation of spontaneous intracerebral hemorrhage using sonothrombolysis. J Neurosurg. 2011; 9. 115(3):592–601. PMID: 21663412.

15. Przybylowski CJ, Ding D, Starke RM, Webster Crowley R, Liu KC. Endoport-assisted surgery for the management of spontaneous intracerebral hemorrhage. J Clin Neurosci. 2015; 11. 22(11):1727–1732. PMID: 26238692.

16. Ritsma B, Kassam A, Dowlatshahi D, Nguyen T, Stotts G. Minimally Invasive Subcortical Parafascicular Transsulcal Access for Clot Evacuation (Mi SPACE) for intracerebral hemorrhage. Case Rep Neurol Med. 2014; 2014:102307. PMID: 25165588.

17. Spiotta AM, Fiorella D, Vargas J, Khalessi A, Hoit D, Arthur A, et al. Initial multicenter technical experience with the Apollo device for minimally invasive intracerebral hematoma evacuation. Neurosurgery. 2015; 6. 11(Suppl 2):243–251. discussion 251. PMID: 25714520.

Fig. 1
(A) Preoperative brain CT, axial view, shows a large left frontal ICH measuring 7.5 × 4.3 × 5.0 cm (volume 80.6 cm3) with intraventricular extension, and resulting in a midline shift of 6.7 mm. The ICH was evacuated through a left eyebrow incision and mini-mOZ craniotomy using the BrainPath endoport system. Postoperative brain CT, (B) 3D reconstruction, shows the mini-OZ craniotomy and, (C) axial view, shows a 4.9 × 1.7 × 2.5 cm residual ICH (volume 10.4 cm3, 87% volume reduction), with significant reduction in midline shift and local mass effect. CT = computed tomography; ICH = intracerebral hemorrhage.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download