Abstract
Objectives
To investigate the rate of postoperative urinary retention (POUR) and identify the risk factors for this complication in women who underwent transvaginal uterosacral suspension surgery.
Methods
A retrospective chart review was conducted for 75 women who underwent transvaginal uterosacral suspension surgery with vaginal hysterectomy, repair of cystocele, and levator myorrhaphy with/without transobturator anti-incontinence surgery. POUR was defined as a need for continuous intermittent catheterization on the third day subsequent to removal of the urethral indwelling catheter.
Results
Acute POUR was reported in 18 women (24.0%). Thirty-six of the 75 patients (48.0%) had undergone anti-incontinence surgery. Crude analysis revealed significant association between the following variables and the risk of POUR: hypertension, the lower average flow rate in the pressure-flow study (PFS), greater post-void residual (PVR) urine volume in PFS, and PVR >30% of the total bladder capacity (TBC) in PFS. In the logistic regression analysis, PVR >30% of the TBC in PFS was identified as the only significant predictor of POUR (odds ratio, 15.4; 95% confidence interval, 2.5–90.9; P = 0.003).
Pelvic organ prolapse (POP) is the most common, causing of hysterectomy among the postmenopausal women who have suffered from genitourinary syndrome of menopause.1 Current treatment for prolapse consists of surgery, conservative management or “watchful waiting”. Mechanical interventions (such as pessaries) and lifestyle interventions (such as weight loss and avoiding placing strain on the pelvic floor) are both conservative management options. In addition, pelvic floor muscle training aims to improve structural support for pelvic organs through effective exercise of the pelvic floor muscles.2 And Acacia nilotica which is a plant contain tannins and steroids is reported to be effective in decreasing the pelvic prolapse, and improving the quality of life.3
Approximately 11% of women undergo surgery for POP or urinary incontinence during their lifetime.4 One of the most common complications of this surgery is postoperative urinary retention (POUR), with an overall rate ranging from 2.5% to 43% depending on the definition used.5678
No valid conclusion regarding voiding dysfunction after prolapse surgery has been drawn from the data available owing to variations in defining and reporting voiding problems. The cutoff level for urinary retention has not been defined by the International Continence Society (ICS). In clinical practice, it is usually defined as a 50 to 200 mL residual volume or >20% of the total bladder volume after voiding. Other studies used the duration of postoperative urethral catheterization as a marker of the presence and severity of urinary retention. The existing evidence is not enough to demonstrate the superiority of any of the definitions.
Early identification and treatment of POUR can prevent further morbidity. However, unknown prolonged bladder distension may result in urinary tract infection (UTI), detrusor dysfunction, and even damage to the surgical repair.9
The purpose of this study was to investigate the incidence of POUR and identify the risk factors of this complication in women who had undergone vaginal prolapse surgery.
This study included all the women who underwent transvaginal uterosacral suspension surgery for symptomatic POP by 1 operator between January 2013 and December 2015. Their electronic medical records were reviewed retrospectively after obtaining Institutional Review Board approval.
We obtained data of baseline demographics, preoperative and postoperative POP-quantification measurements, operative time, estimated blood loss, length of hospital stay, and concomitant operative procedures. Urodynamic study (UDS) was performed for all the women prior to surgery by using the Solar Gold System (Medical Measurement Systems, Dover, NH, USA) after the insertion of gauze packing for prolapse reduction. A multi-channel UDS consists of uroflowmetry, filling cystometry, pressure-flow study (PFS), and urethral pressure profilometry, in accordance with the standard protocol of the ICS recommendations. Preoperative urinalysis and culture were performed to rule out significant bacteriuria or cystitis. All UTIs were treated before surgery.
All the patients underwent hysterectomy. The small bowel was packed out of the operative field with a long gauze, and a Breisky-Navratil retractor was properly positioned to expose the uterosacral ligaments (USLs). The USLs were then bilaterally transfixed in their intermediate portion (at the level of or above the ischial spine plane), with monofilament long-term absorbable 0 sutures (polydioxanone sutures; Ethicon Inc., Piscataway, NJ, USA). The USL sutures were then passed both anteriorly and posteriorly through the peritoneum, apex of the vaginal fascia, and vaginal mucosa. When anterior repair was performed, vesicovaginal fascia plication was performed before fascia transfixion with USL sutures. USL sutures were tightened to close both the peritoneum and vaginal cuff. Internal and external McCall stitches were placed for cul-de-sac obliteration routinely. Levator plication was performed for repair of the rectocele and support of the vaginal vault. Diagnostic cystoscopy was performed after the USL suture to assess ureteral bilateral patency. Transobturator anti-incontinence surgery was decided on the basis of UDS. The indwelling urethral catheter was removed between 2 and 4 days postoperatively in consideration of the patient's willingness.
Acute POUR was defined as the need for continuous intermittent catheterization (CIC) on the third day subsequent to removal of the indwelling urethral catheter. CIC was performed when the post-void residual urine volume was >100 mL or more than one third of the total bladder volume for 2 consecutive times, within 2 days after urethral catheter removal. The patients without POUR served as the comparison group.
Statistical analysis was performed with SPSS Version 16.0 (SPSS Inc., Chicago, IL, USA). A univariate analysis was performed using the Mann Whitney U test, Pearson χ2 test and Wilcoxon signed rank test. A logistic regression analysis was performed to determine the significant parameters of acute POUR after transvaginal uterosacral suspension surgery. Statistical significance was defined as a P value of <0.05.
During the study, 75 patients underwent surgery for symptomatic POP. Eighteen women (24.0%) had POUR. Indwelling of the urethral catheter was prolonged for a mean of 4.6 days (range, 2–6 days). The postoperative CIC was discontinued at a mean postoperative period of 6 days (range, 3–12 days).
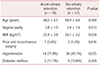
The patients' demographic and baseline characteristics are shown in Table 1. The mean age of the patients in the acute urinary retention group was 68.2 ± 6.5 years. Hypertension was found more often in the acute urinary retention group (77.8%) than in the non-urinary retention group (49.1%; P < 0.05). The remaining characteristics were similar between 2 groups.
The anatomical factors and surgical properties associated with POP are shown in Table 2. The preoperative prolapse stage was similar between 2 groups.
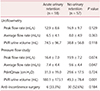
Table 3 presents the comparison of the preoperative UDS between the 2 groups. Post-void residual (PVR) urine volume measured using uroflowmetry was greater in the acute urinary retention group than in the comparison group (74.5 ± 96.7 mL vs. 38.8 ± 56.8 mL; P = 0.118). However, no statistically significant difference in peak or average flow rate was found between 2 groups. In the PFS, the average flow rate was significantly lower in the acute urinary retention group (7.4 ± 4.4 mL/s sv. 9.6 ± 4.4 mL/s; P = 0.047). Furthermore, PVR was greater in the acute urinary retention group than in the non-retention group (180.1 ± 173.1 mL vs. 49.3 ± 78.4 mL; P = 0.001). No statistically significant difference in the proportion of patients who underwent anti-incontinence surgery was found between the 2 groups.
The comparison of preoperative voiding function defined in the UDS is shown in Table 4. PVR >30% of the total bladder capacity (TBC) in PFS was more prevalent in the acute urinary retention group (50.0%) than in the comparison group (10.5%; P = 0.001). The other parameters including PVR >30% of the void volume on uroflowmetry did not show any statistically significant differences between the 2 groups.
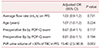
Table 5 shows the results of the multivariate logistic regression analysis for predicting the parameters of acute POUR. PVR >30% of the TBC in PFS was identified as a significant predictor of acute POUR after vaginal reconstructive surgery (odds ratio, 15.4; 95% confidence interval, 2.5–90.9; P = 0.003).
This retrospective study aimed to demonstrate the incidence of acute POUR after surgery for POP and to identify risk factors associated with this complication. Acute urinary retention was found in 24% of the subjects, and PVR >30% of the TBC in PFS was the significant factor that predicted increased risk of POUR.
The incidence of POUR in this study was consistent with those reported in the literature. Hakvoort et al.7 identified a POUR rate of 29% after vaginal POP repair where retention was defined as a PVR volume of >200 mL. Steinberg et al.10 identified a POUR rate of 34% after vaginal mesh procedures for POP where retention was defined as discharge from the hospital with an indwelling Foley catheter due to a failed voiding trial.
The clinical risk factors that affected POUR included age, female sex, lower body mass index, previous incontinence surgery, advanced stage of prolapse and postoperative UTI.1112 The intraoperative risk factors of POUR have been reported as spinal anesthesia, administration of >750 mL of intraoperative fluid, estimated blood loss >100 mL and postoperative opioid use.71314 In addition, a correlation was suggested between the preoperative urodynamic parameters and POUR after prolapse surgery.151617
The clinical usefulness of uroflowmetry has been hampered by the lack of absolute values for defining normal limits. These normal limits would need to be over a wide range of voided volumes, ideally in the form of nomograms. Haylen et al.18 constructed a nomogram of peak and average flow rates on the respective voided volume, known as the Liverpool nomogram. Those with voiding dysfunction show a lower peak or average flow rate.
PVR urine is the volume of urine remaining in the bladder immediately after completion of micturition. A consistently high residual urine volume generally indicates increased outlet resistance, decreased bladder contractility, or both. Absent PVR urine is compatible with normal urinary tract function, but can also exist in the presence of significant filling and storage disorders (incontinence) or with disorders of emptying in which the intravesical pressure is sufficient to overcome increased outlet resistance. What constitutes an abnormally high residual urine volume is not universally established. Previous investigators have empirically chosen volumes of 50 or 100 mL to indicate normal residual urine volumes. However, the residual urine volume is best stated only in the context of total voided volume. Normality should be described as a percentage of the total voided volume. Most asymptomatic women should void spontaneously at least 80% of their total intravesical volume. Abnormal PVR is associated with older age, higher grade of vaginal prolapse and recurrent UTI.
The urodynamic diagnosis of bladder outlet obstruction (BOO) in women is not established contrary to that in men. Even though the normal detrusor pressure at peak flow rate (PdetQmax) is poorly defined, a PdetQmax <10 cm H2O is considered hypotonic and is associated with voiding dysfunction. Blavias-Groutz suggested a nomogram to diagnose BOO in women, with a peak flow rate of <12 mL/sec on uroflowmetry and a PdetQmax of >30 cm H2O.19
In this study, the cutoff peak, and average flow rates, and PdetQmax for predicting POUR were determined by using the Liverpool nomogram and Blavias-Groutz BOO nomogram. In addition, the abnormal PVR that predicts POUR was determined in different clinical settings after uroflowmetry and pressure flow study. All these urodynamic parameters failed to predict POUR after transvaginal uterosacral suspension surgery. Only PVR of more than 30% of the TBC after PFS was revealed as a significant risk factor for predicting acute POUR after transvaginal uterosacral suspension surgery. PVR in PFS after reduction of prolapse with gauze packing could reflect a similar condition as that in postoperative voiding.
The strength of our study was that we incorporated voiding parameters of UDS and clinical factors to identify the predictors of acute POUR after uterosacral suspension surgery. However, our study also had a number of limitations. As a result of the small number of patients, we could not exclude that a lack of statistical power may have impaired our results. In addition, we had no information about longterm voiding function after transvaginal uterosacral suspension surgery.
Our study revealed PVR after PFS with reduced prolapse with gauze packing was a strong predictor of acute POUR. The women with >30% of residual urine volume of TBC in the UDS should be counseled about acute POUR before vaginal prolapse surgery. In addition, this finding of our study can be applied to the clinical management of postoperative urethral catheterization after vaginal prolapse surgery. Further systemic approach for poor voiders who undergo vaginal prolapse surgery is needed.
Figures and Tables
References
1. Kim HK, Kang SY, Chung YJ, Kim JH, Kim MR. The recent review of the genitourinary syndrome of menopause. J Menopausal Med. 2015; 21:65–71.

2. Hagen S, Stark D, Glazener C, Dcikson S, Barry S, Edlers A, et al. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014; 383:796–806.

3. Roozbeh N, Darvish L. Acacia nilotica: New plant for help in pelvic organ prolapse. J Menopausal Med. 2016; 22:129–130.

4. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997; 89:501–506.

5. Tammela T, Kontturi M, Lukkarinen O. Postoperative urinary retention. I. Incidence and predisposing factors. Scand J Urol Nephrol. 1986; 20:197–201.

6. Book NM, Novi B, Novi JM, Pulvino JQ. Postoperative voiding dysfunction following posterior colporrhaphy. Female Pelvic Med Reconstr Surg. 2012; 18:32–34.

7. Hakvoort RA, Dijkgraaf MG, Burger MP, Emanuel MH, Roovers JP. Predicting short-term urinary retention after vaginal prolapse surgery. Neurourol Urodyn. 2009; 28:225–228.

8. Dorflinger A, Monga A. Voiding dysfunction. Curr Opin Obstet Gynecol. 2001; 13:507–512.
9. Geller EJ. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Womens Health. 2014; 6:829–838.

10. Steinberg BJ, Finamore PS, Sastry DN, Holzberg AS, Caraballo R, Echols KT. Postoperative urinary retention following vaginal mesh procedures for the treatment of pelvic organ prolapse. Int Urogynecol J. 2010; 21:1491–1498.

11. Sokol AI, Jelovsek JE, Walters MD, Paraiso MF, Barber MD. Incidence and predictors of prolonged urinary retention after TVT with and without concurrent prolapse surgery. Am J Obstet Gynecol. 2005; 192:1537–1543.

12. Chae JY, Park GY, Kim JH, Kim HJ, Bae JH, Lee JG, et al. Points Aa and Ba are factors associated with preoperative voiding dysfunction in patients with cystocele. Eur J Obstet Gynecol Reprod Biol. 2014; 174:146–149.

13. de Boer HD, Detriche O, Forget P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017; 31:499–504.

14. Keita H, Diouf E, Tubach F, Brouwer T, Dahmani S, Mantz J, et al. Predictive factors of early postoperative urinary retention in the postanesthesia care unit. Anesth Analg. 2005; 101:592–596.

15. Zhang L, Zhu L, Liang S, Xu T, Lang J. Short-term effects on voiding function after mesh-related surgical repair of advanced pelvic organ prolapse. Menopause. 2015; 22:993–999.

16. Lo TS. One-year outcome of concurrent anterior and posterior transvaginal mesh surgery for treatment of advanced urogenital prolapse: case series. J Minim Invasive Gynecol. 2010; 17:473–479.

17. Zhang L, Zhu L, Xu T, Liang S, Lang J. Postoperative voiding difficulty and mesh-related complications after Total Prolift System surgical repair for pelvic organ prolapse and predisposing factors. Menopause. 2015; 22:885–892.

18. Haylen BT, Ashby D, Sutherst JR, Frazer MI, West CR. Maximum and average urine flow rates in normal male and female populations--the Liverpool nomograms. Br J Urol. 1989; 64:30–38.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download