This article has been
cited by other articles in ScienceCentral.
Abstract
This study was conducted to evaluate the effect of Sigma Anti-bonding Molecule Calcium Carbonate (SAC) as therapy for ovariectomy-induced osteoporosis in rats. Three weeks after surgery, fifteen ovariectomized Sprague-Dawley rats were divided randomly into 3 groups: sham-operated group (sham), ovariectomized group (OVX) and SAC-treatment group (OVX+SAC). The OVX+SAC group was given drinking water containing 0.0012% SAC for 12 weeks. Bone breaking force and mineralization as well as blood parameters related to the bone metabolism were analyzed. In OVX animals, blood concentration of 17β-estradiol decreased significantly, while osteocalcin and type I collagen C-terminal telopeptides (CTx) increased. Breaking force, bone mineral density (BMD), calcium and phosphorus in femurs, as well as uterine and vaginal weights, decreased significantly following OVX. However, SAC treatment (0.0012% in drinking water) not only remarkably restored the decreased 17β-estradiol and increased osteocalcin and CTx concentrations, but also recovered decreased femoral breaking force, BMD, calcium and phosphorus, although it did not reversed reproductive organ weights. It is suggested that SAC effectively improve bone density by preventing bone turnover mediated osteocalcin, CTx and minerals, and that it could be a potential candidate for therapy or prevention of postmenopausal osteoporosis.
Keywords: Osteoporosis, bone mineral density, Sigma Anti-bonding Molecule Calcium Carbonate, 17β-estradiol, osteocalcin, type I collagen C-terminal telopeptides
Osteoporosis is defined pathologically as a skeletal disorder characterized by absolute decrease in the amount of bone, leading to fractures after minimal trauma [
1]. Also, osteoporosis is a major age-related health problem for men and women who often have a negative calcium balance due to decreased intestinal calcium absorption, insufficient dietary calcium intake, as well as increased urinary Ca loss associated with estrogen deficiency during menopause [
2]. The main underlying cause of fractures in osteoporosis is increased bone fragility as a result of bone loss. Fracture risk is determined by absolute bone mineral density (BMD), regardless of age [
3-
6]. Low BMD is realized as the most important risk factor that can lead to osteoporosis [
7,
8].
In humans, osteoporosis was classified as 3 types; postmenopausal osteoporosis (Type I), age-related osteoporosis (Type II) and secondary osteoporosis (Type III). Postmenopausal osteoporosis typically affects women by estrogen deficiency within 15 to 20 years after menopause [
9,
10]. BMD of bilateral ovariectomized women is significantly lower than similarly aged women with normal ovarian function [
11]; 50-year-old women who were ovariectomized at about 30 years old have similar bone density as 70-year-old women who become menopausal at the age of 50. This fact indicates that the decline of ovarian function is more important than age [
12]. Therefore, postmenopausal administration of estrogen decreases the occurrence of fractures associated with osteoporosis by about one half [
1].
In patients with osteoporosis, estimation of biochemical markers indicating bone turnover and bone quality has been known to be useful in predicting the rates of bone loss in postmenopausal women [
13-
15]. Bone quality is determined by structural and material properties that are influenced by bone turnover rate [
16]. Histomorphometry is a method of estimating method of bone quality in addition to newer imaging techniques such as microcomputed tomography and magnetic resonance microimaging of bone non-invasively, but the use of these techniques is restricted due to their limited availability, high cost and relatively high radiation exposure [
16]. Accelerated bone turnover leads to the irreversible loss of some trabeculae, resulting in weaker bone and increased risk of fracture [
16]. Therefore, bone turnover is most commonly assessed in clinical practice by the measurement of biochemical markers of bone turnover. Detectable bone turnover markers are products of osteoblast/osteocyst and osteoclast type I collagens (CTx) breakdown reflecting bone formation and bone resorption, respectively [
13,
16]. CTx, and bone type I collagen C-telopeptides, are products of collagen degradation and therefore mirrors bone resorption; elevated CTx levels generally mean accelerated bone turnover [
16]. Therefore, estimation of BMD and CTx may be helpful for predicting fracture risks.
Drugs used in the treatment of osteoporosis can be classified in two groups; antiresorptive or stimulators of bone formation. Antiresorptive drugs include calcium, estrogen, calcitonin and bisphosphonates [
17-
20]. Plant estrogen for osteoporosis treatment has been used for prevention of bone resorption [
21]. But, discontinuation of estrogen administration leads to the recurrence of bone resorption and the degree is similar with bone resorption after ovariectomy. Also, severe side effects of estrogen treatment are endometrial carcinoma, venous thrombosis and pulmonary embolism [
1]. Bisphosphonates are generally well tolerated, with few side effects, which include gastrointestinal symptoms such as nausea, vomiting and diarrhea. However, recent findings suggest that bisphosphonates induce osteonecrosis of the jaw, atrial fibrillation, acute phase response and renal insufficiency, raising safety and ethical concerns around the use of bisphosphonates [
20]. Therefore, new drugs need development for use in the prevention of osteoporosis, particularly in postmenopausal women.
Sigma Anti-bonding Molecule Calcium Carbonate (SAC; activated ionic calcium) harvested from oyster shells as the raw material, results in production of calcium oxide; this calcium has a weak bonding force with other molecules, radicals or atoms. Therefore, this treated calcium can easily be absorbed into the cell, increasing an animal's metabolic system. Also, SAC helps growth and increases endogenous growth hormone by effectively supplying calcium into the cell which is needed for metabolism.
Therefore, this study was performed to evaluate the effect of SAC as a therapy for osteoporosis in an osteoporotic rat model induced by ovariectomy. Also, we investigated the effects of the SAC on BMD, bone and mineral metabolism as well as biochemical markers of bone turnover in osteoporotic rats.
Materials and Methods
Chemicals
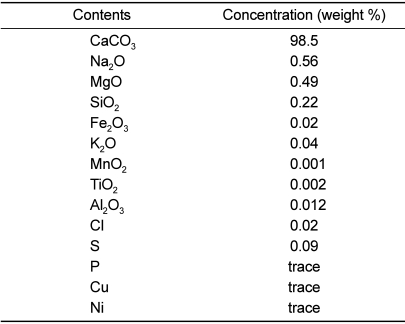
SAC was obtained from NTS Research & Inc. (Coquitlam, BC, Canada). The composition of SAC (above 98% in purity) is shown in
Table 1. Specific gravity (at 20℃) was 7.0-7.5 and heavy metals (As, Pb, Cd and Hg) were <1 ppm.
Animals
Twelve-week old female SPF Sprague-Dawley rats (250 g body weight; n=15) were purchased from the Daehan Laboratory Animal Center (Eumseong, Korea). They were housed in stainless net cages (800W×900L×650H mm) or fenced rooms (1,300W×3,720L×1,250H mm) and 5 rats were contained in each place. The housing conditions as follows: temperature, 23±3℃; relative humidity, 55±10%; lighting time, 12 h (08:00-20:00); ventilation frequency, 10-20 changes/h; illuminance, 150-300 lux. The temperature and humidity was measured hourly by a thermo-hygrometer and there were no variables affecting results of the experiments. The animals were fed a standard rodent chow and purified water ad libitum. All experimental procedures were approved and carried out in accordance with the Institutional Animal Care and Use Committee of Laboratory Animal Research Center at Chungbuk National University, Korea.
Grouping and treatment
Experimental animals were divided randomly into 3 groups of 5 female SD rats. The acclimatized rats underwent either bilateral laparotomy (sham, n=5) or bilateral ovariectomy (OVX, n=10). Three weeks after recovering from surgery, the OVX rats were randomly divided into two groups: vehicle-treated (OVX, n=5) and SAC-treated (OVX+SAC, n=5). Only OVX+SAC rats were given drinking water containing 0.0012% SAC for 12 weeks following 3 weeks post-operation. Changes in body weight and food and water intakes were measured at a regular time. The food and water intakes were calculated by differences between previous intake and the daily measured value.
Blood collection
Blood samples were collected 12 weeks after surgery. Before the blood collection, animals were fasted for 16 h and anesthesia (Zoletile 50®, Virbac, Korea) was given. After an incision in the inguinal region, 0.5 mL of blood was collected from the abdominal artery and kept in a refrigerator (4℃). A part of the blood was used for hematology. Remaining blood was centrifuged at 3,000 rpm for 10 min to obtain serum. The serum was used for blood biochemistry, and for analyses of 17β-estradiol, osteocalcin and CTx which are bone metabolism indicators.
Hematology and blood biochemistry
Hematologic tests were performed using T-540 Coulter Counter (Coulter Electronics Inc, Hialeah, USA) and ADVIA120 Hematology System (Bayer Healthcare LLC, Tarrytown, USA). White blood cells (WBC), differential leukocyte counts, hematocrit, red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelets were analyzed.
The following parameters were analyzed for blood biochemistry (Ciba-Corning 644 Na/K/Cl Analyzer, Ciba-Corning, Medfield, USA): alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), glucose, triglycerides (TG), total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), total proteins (TP), albumin, calcium and phosphorus. As parameters of bone metabolism, serum 17β-Estradiol concentrations were analyzed using a 17β-estradiol kit (Coat-A-Count® Estradiol: Diagnostic Products Corporation, Los Angeles, USA). Osteocalcin levels were determined using a sandwich ELISA kit (Biomedical Technologies Inc., Stoughton, USA), and CTx levels were measured using a serum RatLaps ELISA kit (IDS Inc., Fountain Hills, USA).
Measurement of bone density and minerals
Bone density of the femur was measured using dual energy X-ray absorptionmetry (DEXA; Prodigy Advance, Donga imaging, Korea). Just after BMD measurement, mechanical testing of the femur was performed at room temperature using a materials testing machine (MZ500D; Maruto, Tokyo, Japan). For stabilization, the mid-diaphysis of the femur was placed on two supports of the test apparatus that were 4 mm apart. The load of a three-point bending test was applied in the anteroposterior direction midway between the two supports. The breaking force was calculated using software that measures bone strength (CTR win, Ver 1.05). After breaking force measurement, the femurs were dried at 120℃ for 6 h in a muffle furnace and the dry weights were recorded. The femurs were then incinerated at 800oC for 16 h and the ash was weighed. One hundred milligrams of bone ash was then dissolved in 2 mL of 37% HCl and diluted with distilled water. Calcium and phosphorus contents were determined by atomic absorption spectrophotometry (PerkinElmer; A Analyst 100 Spectrophotometer, Boston, USA).
Statistical analysis
Mean and standard deviation of all measurement values were computed by Microsoft Excel. The significance of differences in the groups was evaluated by ANOVA using Statistical Analysis System (SAS) and were considered significant for values of P<0.05. Post hoc analysis was carried out using Duncan's multiple-range test.
Results
Body weight gain and feed/water intakes
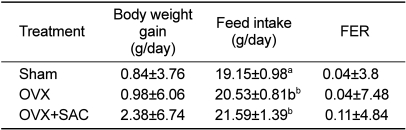
Up to 2 days after the operation, a decrease in body weight was noted in both the OVX and OVX+SAC groups due to post-operative stress but there was consistent increase in body weight and food intake afterwards (data not shown). The increase in body weight after ovariectomy was slightly higher in the ovariectomy group (0.98 g/day) compared to the sham group (0.84 g/day) (
Table 2). Notably, SAC treatment further increased the body weight gain, although there was no statistical significance. In regards to feed intake, significant changes (
P<0.05) in daily feed intake was seen in the OVX group (20.53 g/day) compared to sham group (19.15 g/day). In addition, SAC further enhanced the feed intake, leading to an increased feed efficiency ratio (FER). It is considered that such a difference in feed intake following SAC treatment had an influence on the change in body weight. On the other hand, there were no differences in water intake among groups (data not shown).
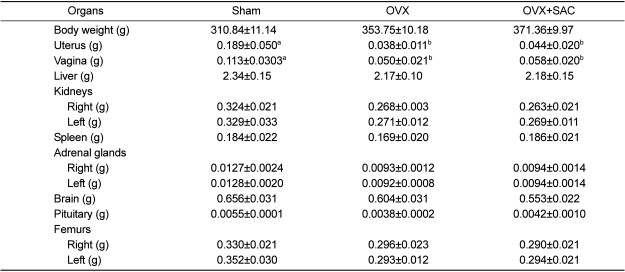
Organ weights
The absolute organ weights are shown in
Table 3. Weights of the reproductive organs (uterus and vagina) were decreased significantly by ovariectomy, which were not recovered by SAC. Other organs including femurs decreased slightly after ovariectomy but there were no statistically significant changes following SAC treatment. It was found that SAC did not have a big influence on organ weights.
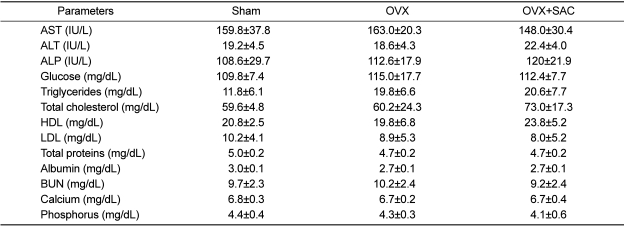
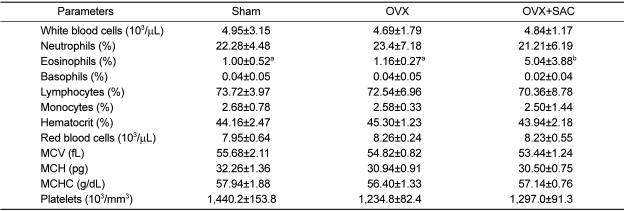
Hematology and blood biochemistry
In hematological analyses,, a statistically significant increase in eosinophils in the SAC treated group after ovariectomy was noted, although there was a big deviation (
Table 4).
There were no significant changes in the blood parameters related to the hepatotoxicity (AST, ALT and ALP), hepatic energy storage and pancreatic injury (glucose), hepatic lipid metabolism (trigltcerides, total cholesterol, DHL and LDL), hepatic protein synthesis (total proteins and albumin), renal injury and electrolyte balance (BUN, calcium and phosphorus) (
Table 5). Especially, however, ALP, a bone growth marker, increased moderately in SAC-treated animals.
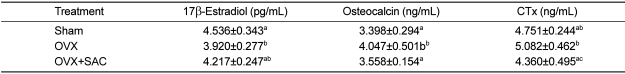
Blood markers of bone metabolism
Blood 17β-estradiol concentration significantly decreased following ovariectomy, and the decrease in 17β-estradiol due to ovariectomy was significantly restored by SAC treatment (
Table 6). Such a result indicates that the decrease in estrogen concentration caused the acceleration of osteoclast generation, accelerating bone resorption, which might be prevented by SAC. Increases in osteocalcin and CTx were found in the ovariectomy group compared to sham group. But such increases in the bone metabolic factors were recovered to their normal levels following treatment with SAC.
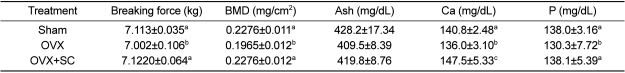
Breaking force and bone mineralization of femurs
The breaking force of femurs of ovariectomized significantly decreased, indicative of the weakened bone integrity (
Table 7). In parallel with the alteration of breaking force, BMD as well as the concentrations of ash, calcium and phosphorus also reduced by ovariectomy. Interestingly, however, the breaking force and related ingredients (BMD, calcium and phosphorus) were fully recovered by SAC treatment.
Discussion
Osteoporosis has been studied by many health professionals and nutritionists in order to prevent the disease. Two major methods are used to diagnose osteoporosis including radiology and biochemistry. Compared to radiology which is a static analysis of bone condition, biochemical diagnosis is able to analyze the dynamic state of the bone by comparing the mutual relationship between markers of bone [
22].
When the weight changes of ovary-removed rats were investigated, the OVX group showed significantly high weight gain compared to the control (sham) group [
9,
23,
24]. Although there was a weight decrease during the first 1-2 days postsurgery caused by stress, weight increased over the 12-week period [
25,
26]. Similar to these results, when we removed both ovaries of the rats, body weights significantly increased in the experimental group. On the other hand, the food and water intake of the control group slightly increased, but was not significantly different. Such results show similarity with previous studies [
25,
26].
It has been attempted to investigate the form of osseous tissue and the kinetics of calcium metabolism by using calcium isotopes. However, because of the complexity of tests and the clinical limitation, a biological marker which has high sensitivity and applicability is needed to measure the bone turnover in osteoporosis. In addition, to evaluate the formation and absorption of the bone matrix, measuring the activity of alkali or acidic oxidase of osteoblasts and osteoclasts can be performed. Also, there is a way of measuring bone matrix components circulating in the system which are released during bone formation and absorption processes.
ALP increased in the serum depending on osteoblastic activity. ALP in the serum indicates positive correlation with other biological markers but has a negative correlation with bone density and it reflects increased bone turnover by bone loss after ovariectomy. However, because the rate of bone turnover can be increased by other diseases such as hyperthyroidism, a true correlation between ALP and osteoporosis may be skewed.
According to Delmas [
27], ALP can be a possible marker for bone turnover but not suitable for bone loss because of the production of the enzyme is not only limited to the osseous tissue; the liver produces an isoenzyme of ALP which reduces the sensitivity and the specificity of the test. In our study, the concentration of the enzyme in serum increased, but did not show relevance with osteoporosis.
Known as a compound closely related with bone metabolism, 17β-estradiol is mainly produced by the ovary, corpus luteum, placenta, adrenal glands and testis. Remarkable fluctuations occur during the menstrual cycle and pregnancy in women. Albright [
28] first mentioned that sex hormone deficiency can cause osteoporosis. Wroolie et al [
29] and Durador et al [
30] reported ovariectomy-induced osteoporosis and menopause induced osteoporosis with significant reductions of 17β-estradiol in the blood. Also in this study, compared to the control group, the experimental group showed significant 17β-estradiol reductions which is consistent to previous studies [
29,
30]. Bone turnover rate is increased by reductions in estrogen, and bone loss rapidly progresses by increased bone absorption compared to bone formation.
Osteocalcin exists in bone and dentin and accounts for about 20% of non-collagen proteins in bone. It was reported that osteocalcin limits mineralization in
in vitro experiments and bone density in mice without the osteocalcin gene is increased [
18]. The concentration of osteocalcin is increased due to the rapid bone turnover rate after menopause but is decreased by injection of estrogen. Therefore, the increase of osteocalcin will likely increase bone turnover rate [
27]. In this experiment after ovariectomy, the concentration of osteocalcin increased, and after the SAC treatment, the concentration remarkably decreased, indicating the changes of bone turnover rate; this suggests the the possibility of SAC as a treatment for osteoporosis.
According to a 2 year period follow-up study among the women who had femoral fractures, the baseline of CTx was increased compared to women without femoral fracture [
13,
16,
31]. Same as in this study, CTx remarkably increased after ovariectomy, followed by a significant decrease with SAC treatment.
Low bone density is a risk factor and tends to progress into decreases in femoral bone density after menopause [
9,
32]. In this study, bone density of the OVX group showed low values compared to the sham group and SAC treatment increased BMD after ovariectomy.
The primary change after estrogen deficiency is an increase in the outflow of calcium from the skeleton. Due to the rapid bone loss, the secretion of parathyroid hormone decreases, causing a decrease in the calcium absorption in the intestine and finally resulting in the loss of calcium from the body and bone [
2,
33]. It was recently reported that over 2-year administration of calcium was more effective in decreasing bone loss of the placebo group in the study of effect of calcium supplement on bone density and fracture in women after menopause with 15 meta-analysis [
17,
18].
In this experiment, compared to the sham group, OVX had a significant decrease in calcium while the OVX+SAC group was not significantly different to the sham group, demonstrating decreased progression to osteoporosis. In summary, osteoporosis induced by ovariectomy or menopause could be alleviated by SAC treatment.
Acknowledgments
This work was supported by the research grant of the Chungbuk National University in 2010.
References
1. Riggs BL. Wyngaarden JB, editor. Osteoporosis. Textbook of Medicine. 1992. 19th ed. Philadelphia: Saunders;p. 1426–1430.
2. Kaplan B, Hirsch M. Current approach to fracture prevention in postmenopausal osteoporosis. Clin Exp Obstet Gynecol. 2004; 31(4):251–255. PMID:
15672957.
3. Brunelli MP, Einghorn TA. Medical management of osteoporosis: fracture prevention. Clin Orthop Relat Res. 1998; 348:15–21. PMID:
9553528.
4. Kleerekoper M. The role of fluoride in the prevention of osteoporosis. Endocrinol Metab Clin North Am. 1998; 27(2):441–452. PMID:
9669148.

5. Bemben DA. Exercise interventions for osteoporosis prevention in postmenopausal women. J Okla State Med Assoc. 1999; 92(2):66–70. PMID:
10024784.
6. Lane JM, Nydick M. Osteoporosis: current modes of prevention and treatment. J Am Acad Orthop Surg. 1999; 7(1):19–31. PMID:
9916187.

7. Hauselmann HJ, Kramer E, Michel BA. Physical therapy in prevention and treatment of osteoporosis. Ther Umsch. 1998; 55(11):724–730. PMID:
9865150.
8. Hough S. Fast and slow bone losers. Relevance to the management of osteoporosis. Drugs Aging. 1998; 12(Suppl 1):1–7. PMID:
9673860.
9. Li M, Shen Y, Wronski TJ. Time course of femoral neck osteopenia in ovariectomized rats. Bone. 1997; 20:55–61. PMID:
8988348.

10. Ohta H, Makita K, Suda Y, Ikeda T, Masuzawa T, Naswa S. Influence of oophorectomy on serum levels of sex steroids and bone metabolism and assessment of bone mineral density in lumbar trabecular bone by QCT-C value. J Bone Miner Res. 1992; 7(6):659–665. PMID:
1414484.

11. Mueller K, Hsiao S. Estrus- and ovariectomy-induced body weight changes: evidence for two estrogenice mechanisms. J comp Physiol Psychol. 1980; 94(6):1126–1134. PMID:
7193690.
12. Richelson LS, Wahner HW, Melton LJ 3rd, Riggs BL. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N Engl J Med. 1984; 311(20):1273–1275. PMID:
6493283.

13. Kim SW, Park DJ, Park KS, Kim SY, Cho BY, Lee HK, Shin CS. Early changes in biochemical markers of bone turnover predict bone mineral density response to antiresorptive therapy in Korean postmenopausal women with osteoporosis. Endocr J. 2005; 52(6):667–674. PMID:
16410657.

14. Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predicts postmenopausal forearm bone loss over 4 years: the OFFLY study. J Bone Miner Res. 1999; 14:1614–1621. PMID:
10469291.
15. Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000; 15:1398–1404. PMID:
10893690.

16. Martin RM, Correa PH. Bone quality and osteoporosis therapy. Arq Bras Endocrinol Metabol. 2010; 54(2):186–199. PMID:
20485908.

17. Fraser LA, Vogt KN, Adachi JD, Thabane L. Fracture risk associated with continuation versus discontinuation of bisphosphonates after 5 years of therapy in patients with primary osteoporosis: a systematic review and meta-analysis. Ther Clin Risk Manag. 2011; 7:157–166. PMID:
21691586.

18. Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf. 2009; 32(3):219–228. PMID:
19338379.
19. Honig S. Osteoporosis, new treatments and updates. Bull NYU Hosp Jt Dis. 2010; 68(3):166–170. PMID:
20969546.
20. Muratore M, Quarta E, Grimaldi A, Calcagnile F, Quarta L. Clinical utility of clodronate in the prevention and management of osteoporosis in patients intolerant of oral bisphosphonates. Drug Des Devel Ther. 2011; 5:445–454.

21. Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW Jr. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998; 68(6 Suppl):1375S–1379S. PMID:
9848502.

22. Price PA, Williamson MK. Origin of the vitamine K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981; 256:12760–12766. PMID:
6975778.
23. Bagi CM, Ammann P, Rizzoli R, Miller SC. Effect of estrogen deficiency on cancellous and cortical bone structure and strength of the femoral neck in rats. Calcif Tissue Int. 1997; 61(4):336–344. PMID:
9312205.

24. Shiraishi A, Higashi S, Masaki T, Saito M, Ito M, Ikeda S, Nakamura T. A comparison of alfacalcidol and menatetrenone for the treatment of bone loss in an ovariectomized rat model of osteoporosis. Calcif Tissue Int. 2002; 71(1):69–79. PMID:
12073154.

25. Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol Behav. 1971; 7(6):847–852. PMID:
5167385.

26. Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinologica. 1973; 72:551–568. PMID:
4739360.

27. Delmas PD, Wilson DM, Mann KG. Effect of renal function on plasma level of bone gla-protein. J Clin Endocrinol Metab. 1983; 57:1028–1030. PMID:
6604733.
28. Albright F. Osteoporosis. Ann Intern Med. 1947; 27(6):861–882. PMID:
18919986.

29. Wroolie TE, Kenna HA, Williams KE, Powers BN, Holcomb M, Khaylis A, Rasgon NL. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17β-estradiol versus conjugated equine estrogens. Am J Geriatr Psychiatry. 2011; 19(9):792–802. PMID:
21873835.

30. Dourador EB, de Falco V, Chahade WH, Cossermelli W, Yoshinari NH. Hormonal and biochemical parameters in postmenopausal osteoporosis. Rev Hosp Clin Fac Med Sao Paulo. 1997; 52(2):60–62. PMID:
9435397.
31. Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandijean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD. Markers of bone resorption predict hip fracture risk in elderly women: the EPIDOS prospective study. J Bone Miner Res. 1996; 11:337–349. PMID:
8852944.
32. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996; 312:1254–1259. PMID:
8634613.

33. Nordin BE, Need AG, Steurer T, Morris HA, Chatterton BE, Horowitz M. Nutrition, osteoporosis, and aging. Ann N Y Acad Sci. 1998; 854:336–351. PMID:
9928442.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download