Abstract
Male factors account for 20% to 50% of infertility cases, and infection in the genitourinary tract may play a contributing role in up to 15% of male infertility. Leukocytospermia is a well-known indicator of infection or inflammation in the male sex glands and the urogenital tract. Although great deal of effort has been expended to elucidate definite management strategies in infertile men with leukocytospermia, the gold standard of treatment remains unclear. Until recently, broad spectrum antibiotics and antioxidants have been used in the treatment of leukocytospermia for male infertility to eliminate infection and reduce reactive oxygen free radicals produced inside cellular mitochondria as a result of inflammation. The present review reveals that antibiotics might improve sperm parameters, the rate of resolution of leukocytospermia, the bacteriologic cure rate, and even the pregnancy rate, although some reports conflict. Antioxidants might also have clinical benefits for sperm function as shown by in vitro studies. However, the data are insufficient to conclude whether antibiotics and antioxidants for the treatment of infertile men with leukocytospermia are effective or not. Better designed investigations into leukocytospermia are needed.
Infertility, defined as the inability of a sexually active, non-contracepting couple to conceive after 1 year of regular intercourse, affects approximately 15% of couples, and male factors are the cause in 20% to 50% of cases [1]. The infectious process in the genitourinary tract may play a contributing role in the reproductive function and fertility in males. Infectious etiologies may be involved in up to 15% of male infertility cases [2]. In particular, leukocytospermia is an abnormal laboratory finding defined by the World Health Organization as the presence of 1×106 leukocytes/mL in human ejaculate and reflects the presence of genital tract infection [3]. Previous studies have demonstrated that leukocytospermia has negative impacts on sperm function and integrity [456]. Aziz et al [4] reported a positive correlation between leukocytospermia and high sperm deformity index scores, acrosomal damage, midpiece defects, and tail deformities.
It is generally accepted that leukocytospermia may indicate infection or inflammation of the male sex glands and urogenital tract [27]. Therefore, broad spectrum antibiotics have been routinely used in the treatment of leukocytospermia for male infertility until recently [8]. In addition, antioxidants that can reduce reactive oxygen species (ROS) produced by semen leukocytes have been used in patients with leukocytospermia [27]. However, there is no clear consensus on the effects of each treatment or on whether leukocytospermia needs to be treated or not. Furthermore, only 1 systematic review of the treatment of leukocytospermia is available, and it was published in 2003 [8].
Therefore, we reviewed the literature regarding the clinical significance and commonly used treatment options, such as antibiotics and antioxidants, of leukocytospermia for male infertility.
MEDLINE (January 1, 1976 to March 1, 2016), Embase (January 1, 1985 to March 1, 2016), the Cochrane Library (January 1, 1987 to March 1, 2016), and KoreaMed (June 1, 1958 to March 1, 2016) were searched with restrictions to English and Korean language publications. The following keywords and MeSH terms were searched through MEDLINE: infertility, male; spermatozoa; anti-bacterial agents; antioxidant; and relevant MeSH terms of pharmacologically active antioxidants, such as vitamins; ascorbic acid; tocopherol; carotene; zinc; selenium; carnitine; fish oils; plant oils; and so forth. Search strategies were adapted for other databases based on the MEDLINE strategy. The bibliographic references of all included trials were reviewed to identify other potentially relevant studies.
We included all randomized controlled trials (RCT) that assessed the efficacy and safety of antibiotics and antioxidants for the treatment of leukocytospermia in male infertility. Non-RCTs and observational studies were excluded. Infertile men with leukocytospermia without any diseases that affected male fertility were included.
The inclusion of all studies was independently decided by two reviewers (JHJ and JK) based on the following selection criteria: study population and intervention. Study selection was made through 2 levels of screening: At the first level, we screened titles and abstracts of identified studies. At the second level, we screened the full text. Any disagreement unresolved by discussion was reviewed by the corresponding author (JTS). Data were extracted from the eligible articles according to predefined criteria: author information, total number of participants, assessment method of leukocytospermia, dose per day, main outcomes, and adverse events. Methodological qualities for each study were assessed by two reviewers (JHJ and JK) independently using the 7-item scale of risk of bias, developed by the Cochrane Bias Methods Group [9]. The six sources of bias assessed were sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. Any unresolved disagreements between reviewers were resolved through discussion or review by the corresponding author (JTS).
The database search found 1,546 potentially eligible articles. After screening abstracts and titles, we excluded 1,083 studies. A total of 30 articles were read in full and evaluated and 11 articles subsequently met the criteria for the systematic review [1011121314151617181920]. Nineteen articles were excluded (non-RCT, 9; duplication, 7; did not meet eligibility, 3) (Fig. 1).
Seven studies included asymptomatic infertile men, but 4 studies included men with accessory gland infection. The included RCTs used a variety of leukocyte assessment methods, such as the monoclonal antibody test, Endtz test, peroxidase stain, and identification of round cells, while 4 studies did not report the leukocyte assessment method [21]. Tetracyclines, quinolones, sulfonamides, macrolides, and penicillins were used for intervention. Wide ranges of doses and treatment durations were applied to the study participants (Table 1).
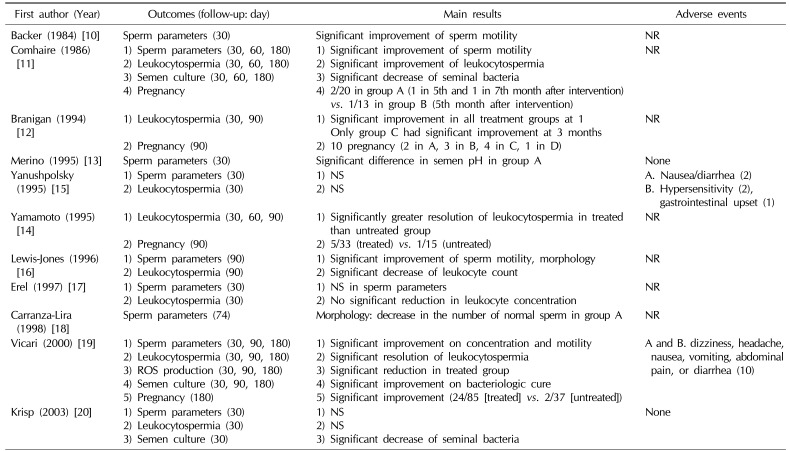
Nine of the 11 studies reported semen analysis after treatment. Three trials showed statistically significant improvement of sperm concentration and/or motility between the treated and untreated group [101119]. However, there were no statistically significant differences in sperm parameters between groups in 3 trials [151720]. Interestingly, Merino and Carranza-Lira [13] found that a statistically significant difference in seminal pH was shown in the group treated with trimethoprim/sulfamethoxazole compared with placebo. In addition, Carranza-Lira et al [18] reported a decrement of the number of normal sperm in the quinolone group compared with the trimethoprim/sulfamethoxazole group. One trial reported significant improvement of sperm motility and morphology using data from all participants who had antibiotic treatment (co-trimoxazole, tetracycline, trimethoprim) (Table 2) [16].
Eight trials reported on the resolution of leukocytospermia after treatment. Antibiotics resulted in a statistically significant resolution of leukocytospermia in 5 trials, in contrast to 3 trials which showed non-significant effects of antibiotics on the resolution of leukocytospermia (significant effect [1112141619]/non-significant effect [151720]). Lewis-Jones et al [16] reported significant resolution of leukocytospermia, but they used data from all participants who had undergone antibiotic treatment. Notably, Branigan and Muller [12] reported that frequent ejaculation (at least every 3 days) with antibiotics was more efficient than antibiotics alone (Table 2).
Four studies reported the pregnancy rate after treatment. Only 1 trial showed statistically significant improvement of the pregnancy rate in the treated group compared with the untreated group, although the overall number of pregnancies was higher in the treatment group (Table 2) (significant improvement [19]/non-significant improvement [111214]).
Adverse events during RCTs were reported in only 4 studies. Yanushpolsky et al [15] noted that 5 patients failed to complete the study due to mild gastrointestinal adverse events and hypersensitivity in the treated group. In addition, Vicari et al [19] also reported 10 mild and transient adverse events in the treatment group. Two studies reported no adverse events in either the treated group or untreated group (Table 2) [1320].
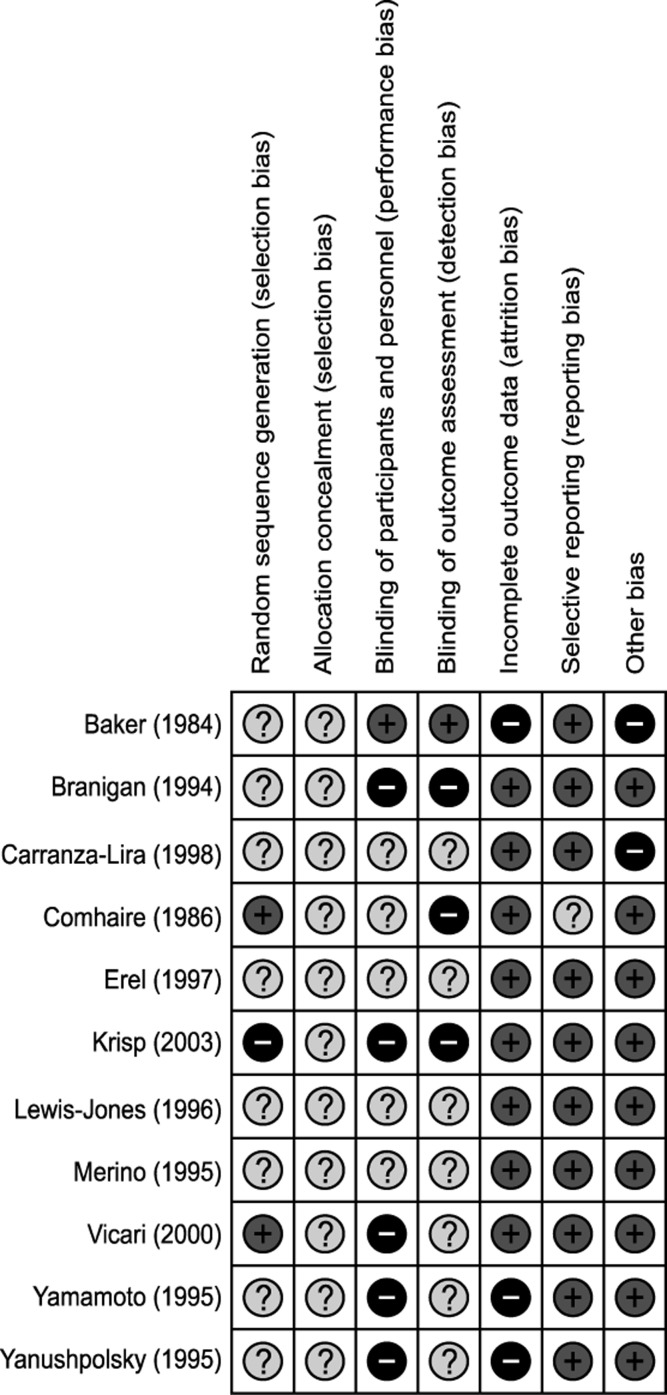
Only two trials had a low risk of bias in random sequence generation. Allocation concealment was not performed in any of trials. All but 1 trial failed to blind the participants and personnel (5: high risk of bias, 5: unclear risk of bias). Blinding of the outcome assessor was also performed inadequately (3: high risk of bias, 7: unclear risk of bias). Due to follow-up loss, a high risk of bias in the incomplete outcome data domain was found in 3 trials. On the other hand, selective reporting and other bias domains were relatively well described (Fig. 2).
Although a great deal of effort has been put into elucidating the best treatment for leukocytospermia, a definite management strategy has not been clearly established. The current management focuses on the elimination of infection and protection from ROS produced inside of cellular mitochondria as a result of inflammation [27]. Based on current best evidence, antibiotics and antioxidants have become the mainstream treatment for leukocytospermia [7].
In 2003, a meta-analysis of 12 studies showed that using broad spectrum antibiotics to treat patients with leukocytospermia might improve sperm concentration, motility, and morphology. However, these studies did not report the pregnancy rate or adverse events, which were the most important primary end points for the infertile patients from the included studies [8]. In our review, sperm parameters and resolution of leukocytospermia were significantly better in the treated group than the untreated group, in addition to a significant decrease in the number of seminal bacteria in the treatment group. However, only 1 of 4 trials showed statistically significant improvement in pregnancy rates in the treatment group [19]. Furthermore, an RCT published in 2003 which was not included in the meta-analysis failed to show the clinical efficacy of antibiotics on sperm parameters or resolution of leukocytospermia [20]. Regarding adverse events, all of the adverse events occurred in the treated group, although these were mild and transient [1519].
Several in vitro studies have also investigated the efficacy of antioxidant therapy for the treatment of leukocytospermia [222324]. Various antioxidants, such as vitamin E, coenzyme Q10, and N-acetyl-L-cysteine, show significantly reduced ROS in human semen and the possibility of improving impaired sperm function [222324]. However, no studies were eligible for our review due to a lack of randomization and/or human studies.
Although some individual studies would contribute to the current evidence on the efficacy of antibiotics and antioxidants, the present review has several limitations from clinical and methodological perspectives. First, the review included few well-designed RCTs. Potentially biased studies exaggerate intervention effects and hence may lead to wrong conclusions. Secondly, among the studies was a great deal of clinical heterogeneity. About half of the studies did not describe the method of diagnosing leukocytospermia. The others used different diagnostic methods for leukocyte counting in semen, such as direct counting, peroxidase stain, and immunocytochemical stain of leukocytes. Direct counting of round cells in semen is highly inaccurate due to the presence of immature germ cells, which cannot be distinguished from leukocytes. Immunocytochemical staining is well-known to be the gold standard for diagnosis of leukocytospermia, but its high cost and the lack of standardization of immunocytochemical staining are the main limiting factors in daily practice [2]. Consequently, the World Health Organization recommends peroxidase staining as the best alternative for diagnosis, although it cannot identify non-peroxidase-rich leukocytes, such as lymphocytes [3]. Interestingly, Barraud-Lange et al [25] showed that leukocytospermia appears to be physiologic at moderate levels (<106/mL) and did not alter the sperm's fertilization ability or clinical pregnancy rates on assisted reproductive technology. In addition, a wide variety of antibiotics with different doses and treatment periods were used in the included trials. Moreover, animal studies showed that antibiotics negatively affected spermatogenesis or sperm parameters by causing spermatogenic arrest in the germ cell not protected from antibiotics by the blood-testis barrier [26]. Therefore, clinicians should bear in mind that an antibiotic's proven effectiveness, even in patients with leukocytospermia, may have detrimental effects on male fertility.
We performed this qualitative review because a meta-analysis of studies with a high risk of bias and considerable heterogeneity might be seriously misleading. There are insufficient data to conclude whether antibiotics and antioxidants for the treatment of infertile men with leukocytospermia are effective or not. Further investigations into leukocytospermia are needed.
The main question for this systematic review was: Is there a benefit from antibiotics or antioxidants for leukocytospermia in terms of human semen quality and fertility? The studies we reviewed showed that antibiotics might improve sperm parameters, the rate of resolution of leukocytospermia, the bacteriologic cure rate, and even the pregnancy rate. Antioxidants had a tendency to improve sperm function in in vitro studies, but the data are insufficient to draw firm conclusions. An effort should be made to establish consistent recommendations for the use of antibiotics and antioxidants in infertile men with leukocytospermia.
ACKNOWLEDGEMENTS
We are grateful to Alea Miller, managing editor of Cochrane Urology Review Group, for her assistance in the preparation of this article.
References
1. Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005; 20:1144–1147. PMID: 15802321.

2. Sandoval JS, Raburn D, Muasher S. Leukocytospermia: overview of diagnosis, implications, and management of a controversial finding. Middle East Fertil Soc J. 2013; 18:129–134.

3. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization;2010.
4. Aziz N, Agarwal A, Lewis-Jones I, Sharma RK, Thomas AJ Jr. Novel associations between specific sperm morphological defects and leukocytospermia. Fertil Steril. 2004; 82:621–627. PMID: 15374705.

5. Lackner JE, Herwig R, Schmidbauer J, Schatzl G, Kratzik C, Marberger M. Correlation of leukocytospermia with clinical infection and the positive effect of antiinflammatory treatment on semen quality. Fertil Steril. 2006; 86:601–605. PMID: 16782098.

6. Tomlinson MJ, White A, Barratt CL, Bolton AE, Cooke ID. The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocytes? Hum Reprod. 1992; 7:517–522. PMID: 1522196.

7. Pentyala S, Lee J, Annam S, Alvarez J, Veerraju A, Yadlapalli N, et al. Current perspectives on pyospermia: a review. Asian J Androl. 2007; 9:593–600. PMID: 17712476.

8. Skau PA, Folstad I. Do bacterial infections cause reduced ejaculate quality? A meta-analysis of antibiotic treatment of male infertility. Behav Ecol. 2003; 14:40–47.

9. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.

10. Baker HW, Straffon WG, McGowan MP, Burger HG, de Kretser DM, Hudson B. A controlled trial of the use of erythromycin for men with asthenospermia. Int J Androl. 1984; 7:383–388. PMID: 6396236.

11. Comhaire FH, Rowe PJ, Farley TM. The effect of doxycycline in infertile couples with male accessory gland infection: a double blind prospective study. Int J Androl. 1986; 9:91–98. PMID: 3539821.

12. Branigan EF, Muller CH. Efficacy of treatment and recurrence rate of leukocytospermia in infertile men with prostatitis. Fertil Steril. 1994; 62:580–584. PMID: 7520396.
13. Merino G, Carranza-Lira S. Infection and male infertility: effect of different antibiotic regimens on semen quality. Arch Androl. 1995; 35:209–212. PMID: 8585775.

14. Yamamoto M, Hibi H, Katsuno S, Miyake K. Antibiotic and ejaculation treatments improve resolution rate of leukocytospermia in infertile men with prostatitis. Nagoya J Med Sci. 1995; 58:41–45. PMID: 7659146.
15. Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ. Antibiotic therapy and leukocytospermia: a prospective, randomized, controlled study. Fertil Steril. 1995; 63:142–147. PMID: 7805903.
16. Lewis-Jones DI, Kynaston HG, Lynch RV, Desmond AD, Entwistle PA. Antibiotic treatment of leucospermia in subfertile males. Arch STD/HIV Res. 1996; 10:65–72.
17. Erel CT, Sentürk LM, Demir F, Irez T, Ertüngealp E. Antibiotic therapy in men with leukocytospermia. Int J Fertil Womens Med. 1997; 42:206–210. PMID: 9222805.
18. Carranza-Lira S, Tserotas K, Morán C, Merino G, Barahona E, Bermúdez JA. Effect of antibiotic therapy in asthenozoospermic men associated with increased agglutination and minimal leukospermia. Arch Androl. 1998; 40:159–162. PMID: 9507749.

19. Vicari E. Effectiveness and limits of antimicrobial treatment on seminal leukocyte concentration and related reactive oxygen species production in patients with male accessory gland infection. Hum Reprod. 2000; 15:2536–2544. PMID: 11098023.

20. Krisp A, Hörster S, Skrzypek J, Krause W. Treatment with levofloxacin does not resolve asymptomatic leucocytospermia: a randomized controlled study. Andrologia. 2003; 35:244–247. PMID: 12950410.
21. Endtz AW. A rapid staining method for differentiating granulocytes from “germinal cells” in Papanicolaou-stained semen. Acta Cytol. 1974; 18:2–7. PMID: 4129934.
22. Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988; 9:367–376. PMID: 3215823.

23. Lewin A, Lavon H. The effect of coenzyme Q10 on sperm motility and function. Mol Aspects Med. 1997; 18(Suppl):S213–S219. PMID: 9266524.

24. Oeda T, Henkel R, Ohmori H, Schill WB. Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility? Andrologia. 1997; 29:125–131. PMID: 9197915.

25. Barraud-Lange V, Pont JC, Ziyyat A, Pocate K, Sifer C, Cedrin-Durnerin I, et al. Seminal leukocytes are Good Samaritans for spermatozoa. Fertil Steril. 2011; 96:1315–1319. PMID: 22047665.

26. Schlegel PN, Chang TS, Marshall FF. Antibiotics: potential hazards to male fertility. Fertil Steril. 1991; 55:235–242. PMID: 1991524.

Fig. 1
Flow diagram of screened, excluded, and analyzed publications based on the inclusion and exclusion criteria. RCT: randomized controlled trials.
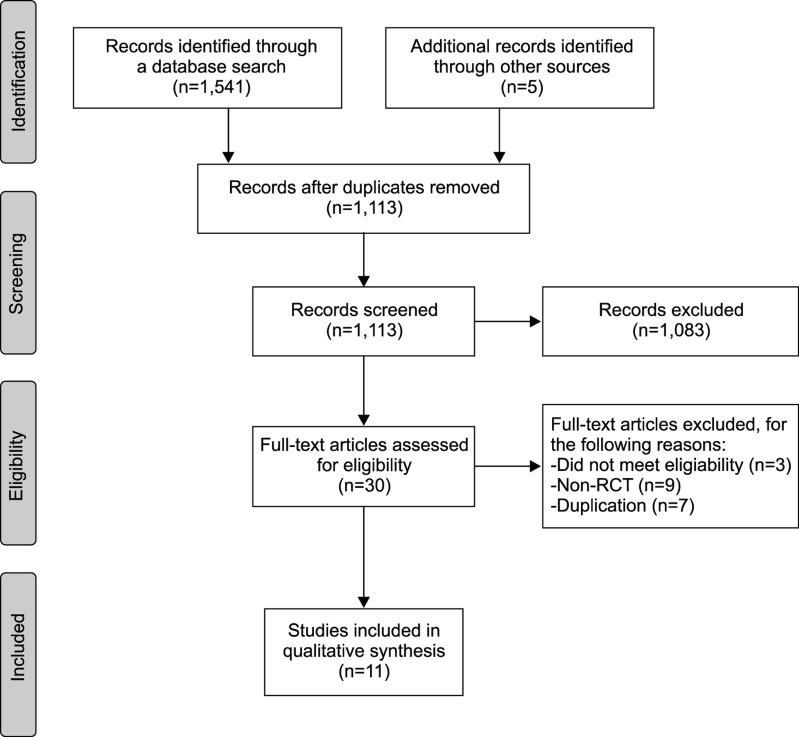
Table 1
Study characteristics of randomized controlled trials of antibiotics for the treatment of leukocytospermia in male infertility
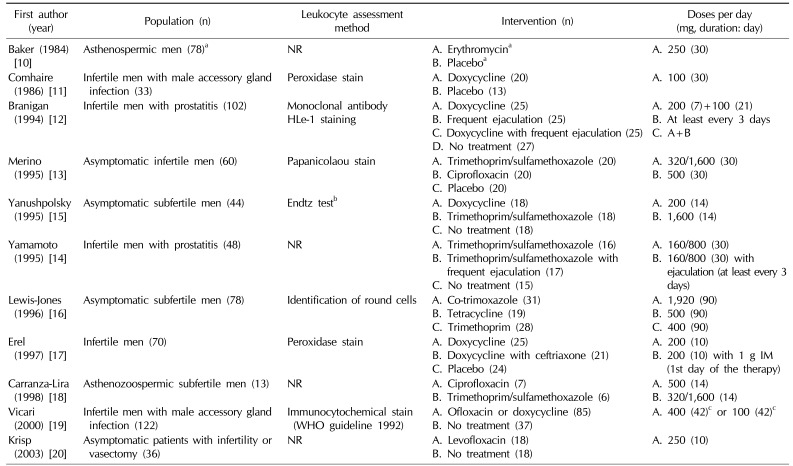
| First author (year) | Population (n) | Leukocyte assessment method | Intervention (n) | Doses per day (mg, duration: day) |
|---|---|---|---|---|
| Baker (1984) [10] | Asthenospermic men (78)a | NR | A. Erythromycina | A. 250 (30) |
| B. Placeboa | ||||
| Comhaire (1986) [11] | Infertile men with male accessory gland infection (33) | Peroxidase stain | A. Doxycycline (20) | A. 100 (30) |
| B. Placebo (13) | ||||
| Branigan (1994) [12] | Infertile men with prostatitis (102) | Monoclonal antibody | A. Doxycycline (25) | A. 200 (7)+100 (21) |
| HLe-1 staining | B. Frequent ejaculation (25) | B. At least every 3 days | ||
| C. Doxycycline with frequent ejaculation (25) | C. A+B | |||
| D. No treatment (27) | ||||
| Merino (1995) [13] | Asymptomatic infertile men (60) | Papanicolaou stain | A. Trimethoprim/sulfamethoxazole (20) | A. 320/1,600 (30) |
| B. Ciprofloxacin (20) | B. 500 (30) | |||
| C. Placebo (20) | ||||
| Yanushpolsky (1995) [15] | Asymptomatic subfertile men (44) | Endtz testb | A. Doxycycline (18) | A. 200 (14) |
| B. Trimethoprim/sulfamethoxazole (18) | B. 1,600 (14) | |||
| C. No treatment (18) | ||||
| Yamamoto (1995) [14] | Infertile men with prostatitis (48) | NR | A. Trimethoprim/sulfamethoxazole (16) | A. 160/800 (30) |
| B. Trimethoprim/sulfamethoxazole with frequent ejaculation (17) | B. 160/800 (30) with ejaculation (at least every 3 days) | |||
| C. No treatment (15) | ||||
| Lewis-Jones (1996) [16] | Asymptomatic subfertile men (78) | Identification of round cells | A. Co-trimoxazole (31) | A. 1,920 (90) |
| B. Tetracycline (19) | B. 500 (90) | |||
| C. Trimethoprim (28) | C. 400 (90) | |||
| Erel (1997) [17] | Infertile men (70) | Peroxidase stain | A. Doxycycline (25) | A. 200 (10) |
| B. Doxycycline with ceftriaxone (21) | B. 200 (10) with 1 g IM (1st day of the therapy) | |||
| C. Placebo (24) | ||||
| Carranza-Lira (1998) [18] | Asthenozoospermic subfertile men (13) | NR | A. Ciprofloxacin (7) | A. 500 (14) |
| B. Trimethoprim/sulfamethoxazole (6) | B. 320/1,600 (14) | |||
| Vicari (2000) [19] | Infertile men with male accessory gland infection (122) | Immunocytochemical stain (WHO guideline 1992) | A. Ofloxacin or doxycycline (85) | A. 400 (42)c or 100 (42)c |
| B. No treatment (37) | ||||
| Krisp (2003) [20] | Asymptomatic patients with infertility or vasectomy (36) | NR | A. Levofloxacin (18) | A. 250 (10) |
| B. No treatment (18) |
NR: not reported, WHO: World Health Organization, IM: intramuscular injection, A, B, C, D: study group according to intervention.
aCross-over study. Seventy-eight patients were randomized (35 had erythromycin then placebo, 5 erythromycin alone, 30 placebo then erythromycin, 8 placebo only). bReference from Endtz AW (Acta Cytol 1974;18:2-7) [21]. cForty days each month for a 3-month period.
Table 2
Main outcomes and adverse events of randomized controlled trials of antibiotics for the treatment of leukocytospermia in male infertility
| First author (year) | Outcomes (follow-up: day) | Main results | Adverse events |
|---|---|---|---|
| Backer (1984) [10] | Sperm parameters (30) | Significant improvement of sperm motility | NR |
| Comhaire (1986) [11] | 1) Sperm parameters (30, 60, 180) | 1) Significant improvement of sperm motility | NR |
| 2) Leukocytospermia (30, 60, 180) | 2) Significant improvement of leukocytospermia | ||
| 3) Semen culture (30, 60, 180) | 3) Significant decrease of seminal bacteria | ||
| 4) Pregnancy | 4) 2/20 in group A (1 in 5th and 1 in 7th month after intervention) vs. 1/13 in group B (5th month after intervention) | ||
| Branigan (1994) [12] | 1) Leukocytospermia (30, 90) | 1) Significant improvement in all treatment groups at 1 Only group C had significant improvement at 3 months | NR |
| 2) Pregnancy (90) | 2) 10 pregnancy (2 in A, 3 in B, 4 in C, 1 in D) | ||
| Merino (1995) [13] | Sperm parameters (30) | Significant difference in semen pH in group A | None |
| Yanushpolsky (1995) [15] | 1) Sperm parameters (30) | 1) NS | A. Nausea/diarrhea (2) |
| 2) Leukocytospermia (30) | 2) NS | B. Hypersensitivity (2), gastrointestinal upset (1) | |
| Yamamoto (1995) [14] | 1) Leukocytospermia (30, 60, 90) | 1) Significantly greater resolution of leukocytospermia in treated than untreated group | NR |
| 2) Pregnancy (90) | 2) 5/33 (treated) vs. 1/15 (untreated) | ||
| Lewis-Jones (1996) [16] | 1) Sperm parameters (90) | 1) Significant improvement of sperm motility, morphology | NR |
| 2) Leukocytospermia (90) | 2) Significant decrease of leukocyte count | ||
| Erel (1997) [17] | 1) Sperm parameters (30) | 1) NS in sperm parameters | NR |
| 2) Leukocytospermia (30) | 2) No significant reduction in leukocyte concentration | ||
| Carranza-Lira (1998) [18] | Sperm parameters (74) | Morphology: decrease in the number of normal sperm in group A | NR |
| Vicari (2000) [19] | 1) Sperm parameters (30, 90, 180) | 1) Significant improvement on concentration and motility | A and B. dizziness, headache, nausea, vomiting, abdominal pain, or diarrhea (10) |
| 2) Leukocytospermia (30, 90, 180) | 2) Significant resolution of leukocytospermia | ||
| 3) ROS production (30, 90, 180) | 3) Significant reduction in treated group | ||
| 4) Semen culture (30, 90, 180) | 4) Significant improvement on bacteriologic cure | ||
| 5) Pregnancy (180) | 5) Significant improvement (24/85 [treated] vs. 2/37 [untreated]) | ||
| Krisp (2003) [20] | 1) Sperm parameters (30) | 1) NS | None |
| 2) Leukocytospermia (30) | 2) NS | ||
| 3) Semen culture (30) | 3) Significant decrease of seminal bacteria |
A,B,C,D: study group according to intervention (A~D of in this Table relate to the Table 1).
ROS: reactive oxygen species, NS: not significant, NR: not reported.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download