Abstract
Purpose
We aimed to investigate the utility of sympathetic skin response (SSR) test for evaluating vasculogenic erectile dysfunction (ED) which is the most common type of impotence.
Materials and Methods
Men in the age group of 28 to 60 years and suffering from vasculogenic ED, as confirmed by a papaverin test and color Doppler sonography, at least for 6 months referred from our university urology department were included. We used the International Index of Erectile Function (IIEF-5) for grading severity of dysfunction and recorded the SSR of every patient from the median, tibial, and dorsal nerves of the penis. One-way analysis of variance (ANOVA), independent t-test and Pearson's correlation coefficient were used for comparing quantitative variables, and Fisher's Exact test was used for comparing qualitative variables. The Mann-Whitney U Test and the Kruskal-Wallis test were performed for analysis of data that were not normally distributed. A p value of less than 0.05 was considered significant.
Results
Forty-two patients were recruited for the study. We found a strong statistical relationship between the IIEF score and the pathologic SSR registered from every mentioned nerve. Patients with abnormal SSR had more severe ED according to IIEF score (p<0.001). In addition, the IIEF score had a significantcorrelation with diabetes mellitus and cardiovascular disease (t-test; p<0.05).
The National Institutes of Health defines erectile dysfunction (ED) as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance [1]. It is the most common sexual disorder among men and negatively influences their intimate relationships, quality of life, and overall self-esteem [2].
ED and coronary artery disease have similar risk factors and pathophysiologic origins, and there is a strong correlation between the degree of ED and the severity of cardiovascular disease. Therefore, ED can be an early sign of cardiovascular disease [2]. ED has different causes, which have been categorized as psychogenic, vasculogenic, neurogenic, hormonal, and drug-induced. The most common cause of ED is vascular, which is subclassified into arterial and venous [3].
A guideline on ED released in 2013 considered neurologic tests such as the bulbocavernosus reflex latency test and the somatosensory evoked potentials test, which assess the somatic nervous system, as part of specific diagnostic tests for ED [4]. Because erections depend on the autonomic system's function, diagnostic tests that assess the autonomic system can be one of the most sensitive tests for ED. The bulbocavernosus reflex is mediated by large-diameter motor fibers, while erection is mediated by small-diameter autonomic fibers. Therefore, bulbocavernosus reflex latency is usually normal in patients with ED. Almost all studies conducted on this subject have suggested autonomic system function tests as sensitive tests for ED diagnosis [5,6,7,8].
In 2001, Zhu and Shen [9] studied sympathetic skin response (SSR) in patients with ED and in normal control subjects and suggested SSR as a new test for diagnosing this problem. Amarenco and Kerdraon [10] studied 19 diabetic subjects and found that for ED diagnosis, the SSR is more sensitive than the bulbocavernosus reflex and other autonomic system function tests. Ashraf et al [11] studied the role of various clinical neurophysiological tests including SSR from limbs, posterior tibial sensory evoked potential, pudendal sensory potential, and bulbocavernous reflex in the evaluation of ED in people with spinal cord disorders and suggested that SSR from the sole was the most sensitive and specific clinical neurophysiological test of ED in this group.
The local SSR at the penis is a useful extension of autonomic testing in ED patients because this method tests the local sympathetic pathway and sometimes, is the only evidence for autonomic deficit. It is noninvasive and evaluates sympathetic small-diameter nerve fibers (postganglionic unmyelinated C fibers), which play the main role in erectile function [12,13]. Furthermore, Valles-Antuña et al [14] identified an indication of the SSR in patients with ED and proposed recording responses not only at classic locations such as the palm of the hand or the sole of the foot but also in the penis.
To the best of our knowledge, thus far, SSR has not been used specifically for autonomic system function assessment in patients of vascular ED. We conducted this study to investigate whether SSR can be helpful for assessing sympathetic function, particularly in patients with vasculogenic ED. We confirmed vasculogenic ED by performing the papaverine test or Doppler sonography, and patients with other problems as the primary causes of ED were excluded [15,16].
This study can not only provide a better understanding of the pathophysiology of this disorder but can also be a basis for future studies using SSR as a tool for treatment monitoring in this population.
We studied men with vasculogenic ED referred from the urology department. These men signed informed consent forms to participate in the study. The research protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences, and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Patients belonging to the age group of 18 to 60 years and suffering from ED for at least 6 months were included in the study; they had an International Index of Erectile Function (IIEF-5) score of 5 to 21, and their ED was confirmed to originate from vascular causes with a papaverine test or Doppler sonography. The exclusion criteria were as follows: 1) use of any drug that may interfere with the SSR or affect erectile function; 2) iatrogenic or traumatic cause for vascular ED; 3) history of previous injury to the median, tibial, or dorsal nerve of the penis or evidence of peripheral neuropathy in electrodiagnosis; and 4) past medical history of chronic renal failure, severe pulmonary disease, or cirrhosis.
We used the Persian version of the IIEF questionnaire, which has already been validated, for investigating the patients' erectile function [15]. The patients' ED was graded as severe for scores of 5 to 7, moderate for 8 to 11, mild-to-moderate for 12 to 16, and mild for 17 to 21. Any patient with a score greater than 21 was considered normal. If the final score for any subject was in the 5 to 21 range, the papaverine test or color Doppler sonography was performed on him to clearly determine the vasculogenic type.
Demographic data including age and marital status; detailed history about medications; previous diseases such as diabetes mellitus, cardiovascular problems (particularly coronary artery disease), hyperlipidemia, and hypertension; any history of blunt perineal or pelvic trauma; pelvic irradiation; and history of cigarette smoking were recorded in a separate questionnaire. We requested laboratory tests to evaluate fasting blood sugar, lipid profile, thyroid function, and total morning testosterone level for all studied men. To exclude any patient with peripheral neuropathy, we performed electrodiagnosis and recorded nerve conduction studies from median, sural, and tibial nerves of both the upper and the lower extremities.
Electrodiagnostic tests were performed using a Medlec Synergy Viasis electromyograph equipped with a bar electrode as a stimulator, two disposable electrodes as recorders, and another disposable electrode as the ground.
For SSR assessment, the electromyography parameters employed in our study were as follows [17]: Sweep speed: 500 ms/D (milliseconds/division); sensitivity: 100 to 1,000 µV/D (microvolts/division); filtering: 0.5 Hz to 2 kHz; stimulation duration: 0.2 ms; stimulation intensity: 10 to 30 mA.
To record SSR, we requested the patients to lie down in the supine position, with their hands beside their bodies in the anatomic position. They were encouraged to be completely relaxed and not to sigh, cough, laugh, or breathe deeply during the test. Skin temperatures during all the tests were 32℃ to 36℃, and all examinations were conducted in rooms with similar constant temperatures of 21℃ to 24℃. After cleaning the site of the electrodes on the skin with alcohol and explaining the test to the subjects, we placed an active recorder electrode (E1) on the palm, 1 cm proximal to the second and third digital web between the second and third metacarpal bones in the upper limb. In the lower limb, it was placed at the plantar surface, 3 cm proximal to the web space between the second and third metatarsal bones. A reference recorder electrode (E2) was placed at the dorsum of the hand in the upper extremity and at the dorsum of the foot in the lower extremity. The ground electrode was located proximal to the E1 electrode with respect to the stimulator cathode's location.
For evaluation of SSR in the penis, E1 was placed on the ventral surface of the shaft, E2 was placed on the dorsal surface of the shaft, and the ground electrode was located at the left iliac crest.
The stimulation was applied at the wrist between the palmaris longus and the flexor carpi radialistendons for the median nerve and posterior to the medial malleolus for the tibial nerve. In the genital area, stimulus was applied at the base of the penis. To prove reproducibility, we repeated stimulation for each nerve three times. To prevent habituation, we maintained an interval of a minimum of 60 s between the application of two stimuli, and the stimuli were applied at irregular intervals. Among the three obtained waves, we attempted to record the wave with the shortest latency for each nerve. If no wave was recorded after ten stimuli, we reported it as absent.
The criteria for considering SSR to be pathologic were as follows: 1) any absent wave; 2) median nerve latency > 1.39 ±0.07 s [17]; 3) tibial nerve latency >1.88±0.11 s [17]; 4) dorsal penile nerve latency >1.5 s [14].
Data were analyzed using PASW Statistics ver. 18.5 (IBM Co., Armonk, NY, USA). One-way analysis of variance (ANOVA) and an independent t-test were performed for comparing the quantitative variables, and the exact Fisher test was performed for comparing the qualitative variables. If the data were not normally distributed, nonparametric statistical tests (Mann-Whitney U test and Kruskal-Wallis test) were performed for analysis. Furthermore, we calculated Pearson's correlation coefficient for testing the correlation between two quantitative variables. A p value of less than 0.05 was considered significant for all tests.
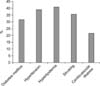
From among the 53 patients tested initially, 11 were excluded after primary evaluation because of iatrogenic or traumatic causes, past medical history of renal failure, and evidence of peripheral neuropathy. Finally, 42 patients were retained for the study. They were 28 to 60 years old, with the mean and median age being 50.8±5.15 and 50 years, respectively. Most patients were married (97.6%). The duration of ED varied between 6 and 36 months, with a mean and median of 15.2±5.15 months and 13 months, respectively. We investigated the risk factors for ED and found that hyperlipidemia was the most prevalent risk factor (Fig. 1).
All 42 subjects had at least one risk factor: 40.5% had one risk factor, 40.5% had two risk factors, and 19% had three risk factors. After measuring the IIEF score of each patient, it was found that 62% had either mild or moderate ED and 38.1% had severe ED (the most prevalent group).
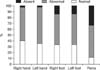
We recorded SSR latencies in both the upper and the lower extremities, as well as in the penis. The SSR latencies of the right median nerves were abnormal in 57.2% of the patients. The left median nerve SSR latencies were abnormal in 61.9% of subjects. Approximately 52.4% of the patients had abnormal SSR latencies in both the left and the right lower extremities. In addition, for 54.8% of the patients, the SSR latencies recorded at the penis were abnormal. We could not record any wave from the median nerves in 2.4% of the subjects, from tibial nerves in 14.3% of the patients, and from dorsal nerves of the penis in 33.3% of the patients (Fig. 2).
The IIEF score had a significant correlation with diabetes mellitus and cardiovascular disease (t-test; p<0.05). However, there was no significant correlation between the IIEF score and hypertension, hyperlipidemia, or cigarette smoking (Table 1). After comparing the groups with normal and abnormal SSR latencies or absent wave, we found a strong significant statistical relationship between the mean IIEF score and SSR latency. Patients with abnormal SSR latencies or absent SSR waves had more severe ED according to their IIEF scores (Table 2). We analyzed the SSR results according to the presence of different cardiovascular risk factors or cardiovascular diseases and found some significant statistical differences in the SSR data of only diabetic and non-diabetic patients (Table 3).
We obtained a negative relationship between age and the IIEF score. Therefore, patients with a greater age had more severe ED, but this relationship was not statistically significant (Pearson's correlation coefficient, r=-0.275; p=0.81).
The main finding of this study was that SSR is an appropriate electrophysiologic test for patients with vasculogenic ED, and its pathology can even reflect the severity of ED. Since 1995, few studies have evaluated the effectiveness of SSR in patients suffering from ED. This might be based on clinicians' assumptions about the obvious relationship between abnormal SSR and ED in general after a few investigations were carried out in this regard. Nonetheless, nobody assessed SSR in specific subgroups of patients with vasculogenic ED. In a study in 2009, the SSRs of 82 patients with ED were registered (with multiple causes: neurological, diabetes, vascular, psychological, and idiopathic), and the mean SSR% in the hand, foot, and penis increased with an increase in the IIEF-5 score. However, there was no statistical correlation between SSR latencies and the results of the IIEF-5 test [14]. Subjects with pathologic SSR (abnormal SSR latency or absent SSR) in our survey had more severe ED according to their IIEF scores.
We recorded pathologic SSR (abnormal SSR latency or absent SSR) in 88.1% of the patients from the dorsal nerves of the penis, the percent which is actually higher than that of patients with pathologic SSR based on recordings from the median or tibial nerves. Therefore, pathologic responses were more frequent in the penis than in the palm or the sole (Fig. 2). This result is in line with the previous findings [9,14]. One study reported a significantly lower mean SSR% registered at the penis than that in the hands and feet [14]. Another study evaluated penile SSR (PSSR) in 20 ED patients aged 22 to 55 years (mean: 31 years; 11 cases occurred after pelvic fractures, 3 after severe injury of the perineum, 1 after fracture of the cervical spine, 1 due to hypertension, 2 with a history of severe masturbation, and 2 without known causes) and reported that PSSR in patients with ED had longer latencies and lower amplitudes compared with the control group [9].
We obtained a negative relationship between age and IIEF score (not statistically significant), and older patients had more severe ED. An increase in the frequency of impotence with age is mainly due to arteriosclerotic changes in the arteries of the penis [18].
ED is one of the most common complications of diabetes mellitus [2]. In our study, 33.3% of the patients had diabetes mellitus (Fig. 1), and the IIEF scores were significantly correlated with the disease state (p=0.001; Table 1). Furthermore, we know that diabetes mellitus frequently causes autonomic dysfunction. The diabetic patients in our sample had a higher rate of abnormality or absence of SSR recorded from the median and the tibial nerves than the non-diabetics. This finding is concordant with the results of a study that proposed the use of SSR for detecting early dysfunction of the small sympathetic fibers in people affected by diabetes mellitus [19].
Cardiovascular disease was present in 23.8% of the subjects included in our study. It had a significant relationship with the IIEF score. ED can be an early sign of cardiovascular disease, and the presence of ED provides a good chance for primary prevention in this population [2].
Finally, we should mention that SSR is a fast, simple, and reproducible technique without pain or discomfort for patients, and it serves as a good choice for the study of diseases involving sympathetic nervous system dysfunction [20,21]. The SSR limitations in clinical practice are mostly linked to the inter- and intra-individual variability of latency and amplitude of the single responses, as well as the phenomenon of habituation [22]. Using repeated stimulations for each nerve, recording the wave with the shortest latency, and applying the stimuli at irregular intervals can reduce the clinical limitations of this easily performed test in patients with vasculogenic ED.
Having no control group is one of the potential study limitations. The risk factors' frequency distribution may not be concordant with previous studies due to the relatively small sample size.
The potential advantage of this study is that we confined the study to subjects with ED resulting from vascular causes by performing color Doppler sonography (an accurate test for vasculogenic impotence diagnosis) and the papaverine test. Our results confirmed the presence of autonomic dysfunction in patients with vasculogenic impotence via the SSR test. The pathologic SSR electrophysiologic test has a significant relationship with the severity of ED, according to the IIEF score.
In future studies, we suggest evaluating the efficacy of the SSR electrophysiologic test in monitoring the response to the ED treatment. Moreover, because subclinical autonomic neuropathy is an early finding in some diseases such as diabetes, we recommend performing the SSR test in diabetic men suffering from vasculogenic ED with and without diabetic neuropathy and comparing these two groups' data in the near future.
Figures and Tables
Fig. 2
Distribution of normal, abnormal, and absent sympathetic skin response in both upper and lower extremities and penis.
ACKNOWLEDGEMENTS
This study is a part of the thesis of Amirhooshang Vahedi (Grant no: 3071). Hence, we would like to thank Shiraz University of Medical Sciences for supporting the research.
References
1. Heidelbaugh JJ. Management of erectile dysfunction. Am Fam Physician. 2010; 81:305–312.
2. Nehra A. Erectile dysfunction and cardiovascular disease: efficacy and safety of phosphodiesterase type 5 inhibitors in men with both conditions. Mayo Clin Proc. 2009; 84:139–148.

3. Wein AJ. Campbell-Walsh urology. 10th ed. Philadelphia: Saunders;2012. p. 677–747.
4. Ryu JK, Cho KS, Kim SJ, Oh KJ, Kam SC, Seo KK, et al. Korean Society for Sexual Medicine and Andrology (KSSMA) guideline on erectile dysfunction. World J Mens Health. 2013; 31:83–102.

5. Ertekin C, Reel F. Bulbocavernosus reflex in normal men and in patients with neurogenic bladder and/or impotence. J Neurol Sci. 1976; 28:1–15.

6. Bird SJ, Hanno PM. Bulbocavernosus reflex studies and autonomic testing in the diagnosis of erectile dysfunction. J Neurol Sci. 1998; 154:8–13.

7. Lavoisier P, Proulx J, Courtois F, De Carufel F. Bulbocavernosus reflex: its validity as a diagnostic test of neurogenic impotence. J Urol. 1989; 141:311–314.

8. Tackmann W, Porst H, van Ahlen H. Bulbocavernosus reflex latencies and somatosensory evoked potentials after pudendal nerve stimulation in the diagnosis of impotence. J Neurol. 1988; 235:219–225.

9. Zhu GY, Shen Y. Sympathetic skin response: a new test to diagnose erectile dysfunction. Asian J Androl. 2001; 3:45–48.
10. Amarenco G, Kerdraon J. Electrophysiologic perineal studies in the exploration of erectile dysfunction in diabetics. Diabete Metab. 1994; 20:60–63.
11. Ashraf VV, Taly AB, Nair KP, Rao S, Sridhar . Role of clinical neurophysiological tests in evaluation of erectile dysfunction in people with spinal cord disorders. Neurol India. 2005; 53:32–35.

12. Daffertshofer M, Linden D, Syren M, Jünemann KP, Berlit P. Assessment of local sympathetic function in patients with erectile dysfunction. Int J Impot Res. 1994; 6:213–225.
13. Derouet H, Jost WH, Osterhage J, Eckert R, Frenzel J, Schimrigk K, et al. Penile sympathic skin response in erectile dysfunction. Eur Urol. 1995; 28:314–319.
14. Valles-Antuña C, Fernandez-Gomez J, Escaf S, Fernandez-Gonzalez F. Sympathetic skin response in patients with erectile dysfunction. BJU Int. 2009; 104:1709–1712.

15. Mehraban D, Shabaninia SH, Naderi GH, Esfahani F. Farsi international index of erectile dysfunction and doppler ultrasonography in the evaluation of male impotence. Iran J Surg. 2006; 14:25–31.
16. Debora M, Daniele A, Alessandro B, Ferri C, Giuseppe M. The role of Doppler ultrasound in the diagnosis of vasculogenic impotence. Arch Ital Urol Androl. 2010; 82:159–163.
17. Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic medicine. 2nd ed. Philadelphia: Hanley & Belfus;2002. p. 252–253.
18. Riedner CE, Rhoden EL, Fuchs SC, Wainstein MV, Gonçalves SC, Wainstein RV, et al. Erectile dysfunction and coronary artery disease: an association of higher risk in younger men. J Sex Med. 2011; 8:1445–1453.

19. Huang YN, Jia ZR, Shi X, Sun XR. Value of sympathetic skin response test in the early diagnosis of diabetic neuropathy. Chin Med J (Engl). 2004; 117:1317–1320.
20. Goizueta-San Martín G, Gutiérrez-Gutiérrez G, Godoy-Tundidor H, Mingorance-Goizueta B, Mingorance-Goizueta C, Vega-Piris L, et al. Sympathetic skin response: reference data for 100 normal subjects. Rev Neurol. 2013; 56:321–326.
21. Emad R, Zafarghasempour M, Roshanzamir S. Sympathetic skin response in incomplete spinal cord injury with urinary incontinence. Ann Indian Acad Neurol. 2013; 16:234–238.

22. Mondelli M, Aretini A, Ballerini M, Vecchiarelli B, Rossi A. Sympathetic skin response. Glabella stimulation may be more useful than peripheral nerve stimulation in clinical practice. Auton Neurosci. 2011; 164:101–104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download