Abstract
Objective
Ovarian carcinosarcoma is a rare subtype of this disease that has not been thoroughly investigated. The aim of this study was to evaluate the prognostic factors and out comes in patients with ovarian carcinosarcoma.
Methods
All patients with histologically confirmed ovarian carcinosarcoma who were treated at Cheil General Hospital and Women's Healthcare Center between January 2000 and December 2015 were identified and analyzed. Data were extracted from medical records, and statistical analyses were performed to determine correlations between clinicopathological parameters and survival outcomes.
Results
Of the 822 patients diagnosed with ovarian cancer over 16 years, 11 (1.3%) had ovarian carcinosarcoma histology. Every patient underwent surgery as the initial treatment followed by intravenous adjuvant chemotherapy. Only 18.1% of cases were early stage (I or II) while 81.8% were advanced stage (III or IV) according to the FIGO (International Federation of Gynecology and Obstetrics) classification. Six cases were of the homologous subtype (54.5%) and five were of the heterologous subtype (45.5%). There was no significant difference in survival according to stage (P=0.24). The heterologous subtype and residual disease were associated with poor disease-free survival (P=0.02 and P=0.04) and overall survival (P=0.02 and P=0.04), On multivariate analysis, the histological subtype was an independent prognostic factor (P=0.02).
Ovarian cancer is the most lethal gynecologic malignancy. Owing to the asymptomatic nature of the disease, more than half of the patients are diagnosed at an advanced stage, resulting in poor prognosis. In 2012, approximately 239,000 women were diagnosed with ovarian cancer worldwide, and 152,000 died of the disease [1]. Among ovarian cancers, carcinosarcoma is a rare histology comprising 1% to 2% of ovarian malignancies, although it is among the most aggressive [23]. Carcinosarcoma, which was previously known as malignant mixed Müllerian tumor, is composed of carcinomatous and sarcomatous elements. Although the pathogenesis of carcinosarcoma is not yet understood, carcinosarcomas are considered metaplastic epithelial carcinomas because most such diseases are of a monoclonal origin [4]. Despite this, the roles of these tumors' sarcomatous elements remain poorly understood. Carcinosarcomas are categorized into homologous and heterologous subtypes based on these sarcomatous elements [5]. The homologous subtype comprises native ovarian tissue consisting of endometrial stromal sarcoma, fibrosarcoma, or leiomyosarcoma. Heterologous elements are tissues that are non-native to the ovary and include cartilage, bone, adipose tissue and smooth or striated muscles. The carcinomatous component is usually serous, endometrioid, clear cell, or squamous cell carcinoma.
Carcinosarcoma of the ovary is considered a disease of poor prognosis compared to serous ovarian cancer; the 5-year survival rates of ovarian carcinosarcoma and serous ovarian cancer are reported to be 28.2% and 38.4%, respectively [6]. However, most investigations of ovarian carcinosarcoma have been through case reports or case series, and the prognostic factors for this tumor type remain unclear because of its rarity. Therefore, this study aimed to evaluate the outcomes and prognostic factors in a cohort of patients with ovarian carcinosarcoma.
All patients with histologically confirmed ovarian carcinosarcoma who were treated at Cheil General Hospital and Women's Healthcare Center between January 2000 and December 2015 were identified. Clinicopathological parameters were extracted from medical and pathology records, which included the following information: age at diagnosis, clinical symptoms, extension of surgery, residual disease, stage, histopathological subtype, adjuvant chemotherapy (number of cycle, regimen), and disease-free and overall survival. Statistical analysis was performed to determine correlation between clinicopathological parameters and survival outcomes.
All patients were staged according to the International Federation of Gynecology and Obstetrics (FIGO) classification based on surgical findings. The histopathological diagnoses were confirmed by a pathologist. All surgeries and chemotherapies were performed by specialists in gynecologic oncology. The surgical procedure was considered complete when it included, at a minimum, a total hysterectomy, bilateral salpingo-oophorectomy, pelvic lymph node dissection, para-aortic lymph node dissection, omentectomy, peritoneal fluid cytology. All other combinations were considered incomplete surgeries. The role of aortic and pelvic lymphadenectomy in patients with advanced ovarian cancer is unclear and has not been addressed by randomized studies [7]. Specifically, in patients with enlarged node on inaccessible location or severe adhesion with vessels, we did not perform lymph node dissection, but did debulking surgery of cancer mass.
Disease-free survival was defined as the time between the last day of first-line chemotherapy administration and the appearance of local recurrence or distant metastases. Patients were followed up every 3 and 6 months during the first 2 years and following 3 years, respectively, using laboratory test including tumor markers and imaging studies. Overall survival was defined as the time from the date of surgery to the date of death or the date of the last follow-up at the outpatient department. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Statistical significance was set at P<0.05. The Cox regression model was used to determine the correlations between clinicopathological parameters and survival outcomes. The statistical analyses were performed using the IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA).
Of the 822 patients over 16 years who were diagnosed with ovarian cancer, 11 (1.3%) had ovarian carcinosarcoma histology. The median patient age was 52 years (range, 42 to 73 years). Six and five patients were postmenopausal (54.5%) and premenopausal (45.5%), respectively. Abdominal distension was the most common symptom and abdominal distention, urinary frequency also noted although 4 patients were asymptomatic. Patient characteristics are shown in Table 1.
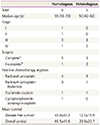
Every patient underwent surgery as initial treatment. Complete surgery was performed in 8 cases (72.7%) including previous hysterectomy due to benign disease; 3 patients (27.3%) underwent incomplete cytoreductive surgery.
The surgical data observed during surgery are shown in Table 2. Only 18.1% of cases were early stage (I or II), while 81.8% were advanced stage (III or IV) according to the FIGO classification (Table 1). Pathology results showed that the homologous subtype was observed in 6 patients (54.5%) and the heterologous subtype in 5 patients (45.5%) (Table 1). Spindle cell sarcoma (n=5) and fibrosarcoma (n=1) were observed in patients with the homologous subtype, while chondrosarcoma (n=3), osteosarcoma (n=1), and cartilage (n=1) were found in those with the heterologous subtype. For the carcinomatous component, serous carcinoma (n=8), endometrioid carcinoma (n=1), mucinous carcinoma (n=1), and poorly differentiated carcinoma (n=1) were observed.
All of the patients received postoperative intravenous adjuvant chemotherapy. The chemotherapy regimen consisted of a platinum-based combination with paclitaxel, ifosfamide, cyclophosphamide, or adriamycin.
The median survival for all patients was 33 months. The 5-year survival rate was 18.1%; 6 patients were still receiving follow-up at the time of this writing. After the first-line chemotherapy, 7 patients experienced a relapse of the disease (63.6%), and one of them died of disease. Five patients underwent second-line chemotherapy, and one received third-line chemotherapy. There were no significant differences between the homologous and heterologous subtype groups in terms of age and the mean number of chemotherapy cycles (8.5±4.0 vs. 7.0±5.3 cycles, mean±standard deviation, respectively).
There was no statistical difference in survival according to stage (P=0.24) and menopausal status (P=0.87). However, the homologous subtype was associated with better disease-free survival (P=0.02) and overall survival (P=0.02) than the heterologous subtype (Table 3, Fig. 1). The presence of residual disease was associated with poorer disease-free survival (P=0.04) and overall survival (P=0.04) (Fig. 2). On multivariate analysis, the Cox regression model using the forward step-wise method showed that histological subtype was an independent prognostic factor (P=0.02), but residual disease was not (P=0.36).
Primary ovarian carcinosarcoma is a rare disease. We found that carcinosarcomas represent only 1.3% of the total number of ovarian cancer patients treated at our institution over a 16-year period, which is a similar rate to that previously reported [8]. The prognostic factors of ovarian carcinosarcoma are not yet well established because of the rarity of this disease. Some possible factors such as age and menopausal status have been proposed [59]; however, these were not found to be associated with disease prognosis in the current study. The median age (53 years) in our study was younger than that in previous studies, which might have influenced our result [1011]. Moreover, carcinosarcoma of the ovary occurred in both postmenopausal and premenopausal women, although there was no significant difference in survival according to menopausal status.
The prognostic significance of the FIGO stage for carcinosarcoma has been previously demonstrated [1112], as was the survival advantage for patients with early-stage disease. However, at the time of primary treatment, patients with ovarian carcinosarcoma usually present with advanced-stage disease. In this study, only 18.1% of patients were diagnosed at an early stage while 81.8% were at an advanced stage, which is consistent with previous studies. However, it was impossible to determine the prognostic value of disease stage in this study because of the low statistical power due to the small number of early-stage cases.
It is well known that optimal cytoreductive surgery is associated with improved survival in ovarian carcinosarcoma patients [13]. Consistent with this, our study also showed that patients who underwent optimal cytoreduction without gross residual disease tended to exhibit better disease-free and overall survival times than those with residual disease after surgery.
The prognostic value of the histological subtype is controversial. Several previous studies showed that the type of the sarcomatous element (i.e., heterologous vs. homologous) is a prognostic factor [141516]. Furthermore, a previous study showed that progression of ovarian carcinosarcoma was associated with epithelial-to-mesenchymal transition. Upon recurrence, the metastatic lesion exhibited a greater proportion of sarcomatous elements than the primary debulked lesion [17]. Based on these results, we hypothesized that the carcinomatous components initiate tumorigenesis while the sarcomatous components drive disease progression. In our study, the sarcomatous component was a significant predictor of disease recurrence and survival time; all early-stage patients showed homologous sarcomatous components, who in turn showed more favorable disease-free and overall survival times. Taken together, these data indicated that histologic subtype and residual disease after surgery both affect survival. However, histologic subtype was the exclusive independent prognostic factor in this study.
Owing to the rarity of this type of cancer, the most effective chemotherapy regimen has not yet been determined. Since the combination of paclitaxel and carboplatin is an effective regimen for epithelial ovarian cancer; this regimen may also be of benefit for the treatment of ovarian carcinosarcomas after cytoreductive surgery [161819]. In our study, 7 patients received a paclitaxel and platinum combination regimen as first-line chemotherapy; their median disease-free and overall survival times were 26.5 and 33 months, respectively. In contrast, 2 patients who received an ifosfamide and platinum combination regimen achieved 52.5 and 52.5 months of median disease-free and overall survival times, respectively. However, the small number of patients limited our ability to discover better regimens for the treatment of this disease.
Ovarian carcinosarcoma is a rare malignancy and has an aggressive clinical course. Nonetheless, optimal cytoreduction without gross residual disease and a homologous subtype are favorable prognostic factors in terms of disease relapse and survival. In this study, we strove to clarify the prognostic factors of this disease with a larger group of patients than previously published, although our cohort was still relatively small given the rarity of the disease. Future studies that explore additional prognostic factors and novel treatment agents in even larger cohorts are warranted.
Figures and Tables
Fig. 1
Kaplan-Meier survival curves according to the histopathological subtype. (A) Disease-free survival and (B) overall survival.

Fig. 2
Kaplan-Meier survival curves according to the presence of a residual mass. (A) Disease-free survival and (B) overall survival.

Table 1
Characteristics of patients with ovarian carcinosarcoma
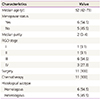
Table 2
Surgical data of patients with carcinosarcoma of the ovary

Table 3
Comparison of clinical and pathologic data according to the histological subtype of ovarian carcinosarcoma
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
2. Silasi DA, Illuzzi JL, Kelly MG, Rutherford TJ, Mor G, Azodi M, et al. Carcinosarcoma of the ovary. Int J Gynecol Cancer. 2008; 18:22–29.
3. George EM, Herzog TJ, Neugut AI, Lu YS, Burke WM, Lewin SN, et al. Carcinosarcoma of the ovary: natural history, patterns of treatment, and outcome. Gynecol Oncol. 2013; 131:42–45.
4. Jin Z, Ogata S, Tamura G, Katayama Y, Fukase M, Yajima M, et al. Carcinosarcomas (malignant mullerian mixed tumors) of the uterus and ovary: a genetic study with special reference to histogenesis. Int J Gynecol Pathol. 2003; 22:368–373.
5. Harris MA, Delap LM, Sengupta PS, Wilkinson PM, Welch RS, Swindell R, et al. Carcinosarcoma of the ovary. Br J Cancer. 2003; 88:654–657.
6. Rauh-Hain JA, Diver EJ, Clemmer JT, Bradford LS, Clark RM, Growdon WB, et al. Carcinosarcoma of the ovary compared to papillary serous ovarian carcinoma: a SEER analysis. Gynecol Oncol. 2013; 131:46–51.
7. Iwase H, Takada T, Iitsuka C, Nomura H, Abe A, Taniguchi T, et al. Clinical significance of systematic retroperitoneal lymphadenectomy during interval debulking surgery in advanced ovarian cancer patients. J Gynecol Oncol. 2015; 26:303–310.
8. Bicher A, Levenback C, Silva EG, Burke TW, Morris M, Gershenson DM. Ovarian malignant mixed mullerian tumors treated with platinum-based chemotherapy. Obstet Gynecol. 1995; 85:735–739.
9. Brown E, Stewart M, Rye T, Al-Nafussi A, Williams AR, Bradburn M, et al. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer. 2004; 100:2148–2153.
10. Morrow CP, d'Ablaing G, Brady LW, Blessing JA, Hreshchyshyn MM. A clinical and pathologic study of 30 cases of malignant mixed mullerian epithelial and mesenchymal ovarian tumors: a Gynecologic Oncology Group study. Gynecol Oncol. 1984; 18:278–292.
11. Chang J, Sharpe JC, A'Hern RP, Fisher C, Blake P, Shepherd J, et al. Carcinosarcoma of the ovary: incidence, prognosis, treatment and survival of patients. Ann Oncol. 1995; 6:755–758.
12. Muller M, Dupre PF, Lucas B, Simon H, Malhaire JP, Guillemet C, et al. Carcinosarcoma of the ovary. J Gynecol Obstet Biol Reprod (Paris). 2007; 36:399–402.
13. Rauh-Hain JA, Growdon WB, Rodriguez N, Goodman AK, Boruta DM 2nd, Schorge JO, et al. Carcinosarcoma of the ovary: a case-control study. Gynecol Oncol. 2011; 121:477–481.
14. Loizzi V, Cormio G, Camporeale A, Falagario M, De Mitri P, Scardigno D, et al. Carcinosarcoma of the ovary: analysis of 13 cases and review of the literature. Oncology. 2011; 80:102–106.
15. Sood AK, Sorosky JI, Gelder MS, Buller RE, Anderson B, Wilkinson EJ, et al. Primary ovarian sarcoma: analysis of prognostic variables and the role of surgical cytoreduction. Cancer. 1998; 82:1731–1737.
16. Mok JE, Kim YM, Jung MH, Kim KR, Kim DY, Kim JH, et al. Malignant mixed Mullerian tumors of the ovary: experience with cytoreductive surgery and platinum-based combination chemotherapy. Int J Gynecol Cancer. 2006; 16:101–105.
17. Amant F, Vloeberghs V, Woestenborghs H, Moerman P, Vergote I. Transition of epithelial toward mesenchymal differentiation during ovarian carcinosarcoma tumorigenesis. Gynecol Oncol. 2003; 90:372–377.
18. Leiser AL, Chi DS, Ishill NM, Tew WP. Carcinosarcoma of the ovary treated with platinum and taxane: the memorial Sloan-Kettering Cancer Center experience. Gynecol Oncol. 2007; 105:657–661.
19. Muntz HG, Jones MA, Goff BA, Fuller AF Jr, Nikrui N, Rice LW, et al. Malignant mixed mullerian tumors of the ovary: experience with surgical cytoreduction and combination chemotherapy. Cancer. 1995; 76:1209–1213.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download