Abstract
Growing teratoma syndrome (GTS) is an increase in tumor size containing only mature teratoma component, during or after chemotherapy for germ cell tumors. A surgical resection is important to confirm diagnosis and is considered as the most appropriate therapeutic management. GTS is rare event in association with ovarian germ cell tumors. In this report, we present a case of GTS which occurred following the treatment of mixed ovarian germ cell tumor with immature teratoma and endodermal sinus tumor. The patient was given adjuvant chemotherapy consisting of six cycles of etoposide and cisplatin. Abdomen pelvis computed tomography performed after chemotherapy demonstrated no evidence of recurrence. She is on regular follow up and remained disease-free for 8 months following second surgery.
Growing teratoma syndrome (GTS) is defined as an increase in tumor size in a patient with germ cell tumor during or after chemotherapy while tumor markers are normal and histology shows only mature teratoma. Logothetis et al. [1] first used this term in 1982. DiSaia et al. [2], in 1977, described this phenomenon as "chemotherapeutic retroconversion". Later it was understood that the two terminologies were synonymous [3]. GTS appears in 1.9-7.6% after chemotherapy in testicular non-seminomatous germ cell tumors [4]. But GTS originating from ovarian germ cell tumor is very rare [5].
In this report, we present the case of a 17-year-old GTS patients following treatment of ovarian mixed germ cell tumors.
A 17-year-old female patient presented to gynecologic clinic in Mexico with a history of intermittent abdominal pain and abdominal distension. Abdominopelvic (AP) computed tomography (CT) demonstrated 14 × 9 × 16 cm sized cystic and solid mass in the pelvic and abdominal cavity with calcification. And CT demonstrated ascites and omental infiltration and peritoneal thickening and no evidence of lymph node or hematogenous metastasis (Fig. 1). CA-125 was 159.0 U/mL and alpha fetoprotein (AFP) was 834.5 ng/mL. Exploratory laparotomy revealed a 20 cm sized right ovarian tumor. Right ovarian cystectomy and peritoneal tissue multiple biopsies were performed. Pathology revealed mixed germ cell tumor with grade III immature teratoma (Fig. 2) and endodermal sinus tumor (yolk sac tumor) (Fig. 3). Multiple peritoneal biopsies were positive for malignancy. She was therefore diagnosed to have mixed germ cell tumor, stage IIIc. After surgery, the patient was referred to our hospital. AP magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT), done before chemotherapy, revealed residual cancers in the abdominal cavity. About 5 × 5 cm sized Right ovarian mass and 6×5cm sized Left ovarian mass and peritoneal carcinomatosis with metastatic lymph nodes in left paraaortic and iliac bifurcation and right common iliac area were seen. CA-125 was 75 U/mL and AFP was 149 ng/mL. Six cycles of combination chemotherapy with bleomycin, etoposide and cisplatin were given every three weeks. Following chemotherapy, tumor markers returned to within normal limits, CA-125 was 0.5 U/mL and AFP was 1ng/mL. But the AP MRI demonstrated no interval change of residual cancer in both ovaries with peritoneal carcinomatosis (Fig. 4). She was followed up without further treatment.
Because she was young, and following tumor markers was normal and following AP MRI, there was no interval change of both ovarian mass size.
The secondary laparotomy was performed 16 months following last chemotherapy because of persistent radiographic evidence of disease. The purpose of operation was diagnosis and removal of tumor. Even more than before chemotherapy, Abdominal and pelvic examination during laparotomy revealed growing masses of both ovarian tumors. At laparotomy, 6 × 4 cm sized right ovarian tumor and 7 × 4.5 cm sized left ovarian tumor were found. In addition, 10 × 6 cm sized diaphragmatic mass and 2-5 cm sized peritoneal mass were found. And severe adhesion in abdominal and pelvic cavity, both ovary were adherent to lateral pelvic wall and uterus was adherent to rectum and posterior cul-de-sac obliteration due to tumor plaques. And tumor of diaphragm was adherent to liver surface. For diagnosis, Frozen biopsies of omentum and peritoneal mass were done. The histological examination revealed the masses of biopsies to be teratoma. Total hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection, total omentectomy, appendectomy, tumorectomy and diaphragmatic mass excisions were performed. After operation, there were 1-2 mm miliary seeding nodules on peritoneal surface. Final pathology revealed that tumor consisted of mature tissue including mature glial, epithelial tissue and cartilage. And tumor presented in bilateral ovary, peritoneum, mesoappendix, omentum and diaphragm. The patient was given adjuvant chemotherapy consisting of six cycles of etoposide and cisplatin after debulking surgery. AP CT showed interval decrease in size of the calcified solid nodule in the pelvic cavity and tumor markers were maintained normal range recommending regular follow-up. The patient is on regular follow up at outpatient clinic without evidence of recurrent disease for eleven months.
GTS is an extremely rare complication of malignant germ cell tumor, especially in females [1].
The definition of GTS requires three criteria [1]. 1) Clinical or radiological enlargement of tumors during or after chemotheraphy administered for nonseminomatous germ cell tumors (NSGCT); 2) Normalization of previously elevated tumor markers (AFP or β-human chorionic gonadotropin); and 3) Absence of any NSGCT component other than mature teratoma at histological examination of the entirely resected tumor.
Treatment depends on the age of the patient and the specific tumor type. Although surgery is the most common form of treatment for immature teratoma, extensively destructive surgery, including total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, lymph node sampling and appendectomy is seldom considered for immature teratoma, except for those patients who have completed their fertility. Fertility-preserving surgery (removing the affected ovary and preserving the contralateral ovary and the uterus) followed by combination chemotherapy has become the standard of care of early stages and selected advanced ovarian GCTs [6]. Since 1989, bleomycin, etoposide, and cisplatin combination has become the standard chemotherapy treatment for all ovarian GCTs Three or four courses of chemotherapy have placed patients into remission with long-term survival.
In this report, we present the case of chemotherapeutic retroconversion of residual tumor of mixed ovarian germ cell with immature teratoma and endodermal sinus tumor after postoperative chemotherapy. The presented case reports persistent radiographic evidence of disease in the face of normal serum tumor markers. The normalization of AFP generally signifies cure for endodermal sinus tumors and mixed germ cell tumors contained endodermal sinus components, particularly if the AFP remains normal over an extended period of time [7]. Decreasing of the AFP value below the normal range is recognized as an indication of remission [8]. After second laparotomy, histologically, the residual masses contained mature teratoma with no elements of immature components.
Incomplete resection of the primary tumor is considered to be one of the risk factors for GTS. Andre et al. analysis cases of 30 male patients of GTS, one patient (4%) having undergone complete resection (n = 24) had recurrent GTS, compared all but one patient (83%) in whom resection was partial (n = 6) [9]. The other risk factors for GTS were histological grade 2 or 3 and clinical stage III or IV. Despite relative benign characteristics of GTS, two types of clinical complications can be observed in GTS. First, mechanical complications can occur because of the tumor growth which can compress the surrounding organs. Second, malignant transformation of GTS can occur in 3% of the cases [9]. The transformation could be into immature teratoma, sarcoma, adenocarcinoma, squamous cell carcinoma, or primitive neuroectodermal tumor. Therefore histologically mature teratoma may have benign appearances but with potential malignancy [10].
The treatment of GTS is surgical resection as complete as possible for several reasons. First reason is to confirm diagnosis and to exclude malignancy. Second is to relieve possible pressure mass effects from adjacent organs. Third is to prevent malignant transformation. Most of the patients are then in complete remission and no further treatment is indicated.
The patient was given adjuvant chemotherapy consisting of six cycles of etoposide and cisplatin. Surgery and adjuvant platinum based chemotherapy in GTS have been reported to give a 5-year survival rate of 93% [11].
The mechanism of the evolution from malignant germ cell tumor to mature teratomatous tissue in not fully understood. The chemotherapeutic agent used may induce somatic maturation in the malignant cell [12]. Or, the malignant elements can be destroyed by the chemotherapy. In conclusion, complete surgical resection of GTS is necessary to prevent complication and recurrence of GTS and malignant transformation. But, there is no standard approach with high dose chemotherapy after surgery from clinical experience. So, the effect of postoperative chemotherapy in GTS need further study. Although GTS has a good prognosis, close follow-up with tumor markers and imaging studies are highly recommended, because stabilization of GTS can take as long as 10 years after diagnosis [13]. At the time of this report, Normalization of tumor markers and no evidence of recurrence has been observed in our patient.
Figures and Tables
Fig. 1
Abdomen pelvic computed tomography shows about 14.1 × 9.1 cm sized huge cystic and solid mass in the pelvic and abdominal cavity with fine calcification.
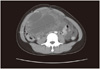
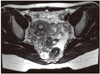
Fig. 2
Immature teratoma showing squamous epithelial component and immature mesenchymal stromal component (H&E, ×200).

References
1. Logothetis CJ, Samuels ML, Trindade A, Johnson DE. The growing teratoma syndrome. Cancer. 1982. 50:1629–1635.
2. DiSaia PJ, Saltz A, Kagan AR, Morrow CP. Chemotherapeutic retroconversion of immature teratoma of the ovary. Obstet Gynecol. 1977. 49:346–350.
3. Amsalem H, Nadjari M, Prus D, Hiller N, Benshushan A. Growing teratoma syndrome vs chemotherapeutic retroconversion: case report and review of the literature. Gynecol Oncol. 2004. 92:357–360.
4. Maroto P, Tabernero JM, Villavicencio H, Mesía R, Marcuello E, Solé-Balcells FJ, et al. Growing teratoma syndrome: experience of a single institution. Eur Urol. 1997. 32:305–309.
5. Kattan J, Droz JP, Culine S, Duvillard P, Thiellet A, Peillon C. The growing teratoma syndrome: a woman with non-seminomatous germ cell tumor of the ovary. Gynecol Oncol. 1993. 49:395–399.
6. Tzortzatos G, Sioutas A, Schedvins K. Successful pregnancy after treatment for ovarian malignant teratoma with growing teratoma syndrome. Fertil Steril. 2009. 91:936.e1-3.
7. Razzi S, Luisi S, Gabbanini M, Lazzeri L, Mazzini M, Petraglia F. Yolk sac tumor in a young girl: a case report. Gynecol Endocrinol. 2005. 20:334–335.
8. Chen CH, Yang MJ, Cheng MH, Yen MS, Lai CR, Wang PH. Fertility preservation with treatment of immature teratoma of the ovary. J Chin Med Assoc. 2007. 70:218–221.
9. André F, Fizazi K, Culine S, Droz J, Taupin P, Lhommé C, et al. The growing teratoma syndrome: results of therapy and long-term follow-up of 33 patients. Eur J Cancer. 2000. 36:1389–1394.
10. Sella A, el Naggar A, Ro JY, Dexeus FH, Amato RJ, Lee JS, et al. Evidence of malignant features in histologically mature teratoma. J Urol. 1991. 146:1025–1028.
11. Tewari K, Cappuccini F, Disaia PJ, Berman ML, Manetta A, Kohler MF. Malignant germ cell tumors of the ovary. Obstet Gynecol. 2000. 95:128–133.
12. Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev. 2008. 34:427–441.
13. Hsieh TY, Cheng YM, Chang FM, Chou CY. Growing teratoma syndrome: an Asian woman with immature teratoma of left ovary after chemotherapy. Taiwan J Obstet Gynecol. 2009. 48:186–189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download