Introduction
Depression during pregnancy is highly prevalent, affecting between 10% and 16% of pregnant women. Treatment of depression during the antenatal period is essential; if untreated, a woman may not seek optimal prenatal care, and may be more likely to make poor lifestyle choices, including smoking and using alcohol. The result is an increased risk of premature birth, low birth weight, and other neonatal complications, as well as an increased risk of maternal postpartum depression and difficulty in forming a bond with the baby [
1].
Antidepressant use is common among women of childbearing age. Three to six percent of pregnant women use selective serotonin reuptake inhibitors (SSRIs); however, the safety of frequently prescribed SSRI antidepressants in pregnancy is under debate [
2]. It has been suspected that the use of some SSRIs during pregnancy is associated with congenital anomalies, neonatal withdrawal, and attention deficit hyperactivity disorder. Citalopram is an SSRI commonly prescribed during pregnancy. Several reports have suggested a link between maternal exposure to citalopram and congenital malformations, particularly cardiac malformations [
34567891011]. Suspected major malformations that have been linked to antenatal citalopram use include neural tube defects (odds ratio [OR], 2.46; 95% confidence interval [CI], 1.20 to 5.07) [
10], cardiac septal defects (OR, 2.52; 95% CI, 1.04 to 6.10) [
6], patent ductus arteriosus (OR, 5.5; 95% CI, 2.3 to 13.6) [
7], and birth defects previously identified to be associated with SSRI use, such as anencephaly, craniosynostosis, and omphalocele (OR, 4.0; 95% CI, 1.3 to 11.0) [
3]. Despite these potential safety issues, only one meta-analysis concerning the safety of citalopram use in pregnancy showed that there was no association between major congenital and cardiac malformations and antenatal citalopram use [
12]. However, descriptions of the included studies were of poor quality and unspecific.
Therefore, the objective of this meta-analysis was to systematically update and describe the available relevant data, and to evaluate the effects of maternal exposure to citalopram regarding associated congenital anomalies. Special attention was given to the possible association between the use of citalopram and the prevalence of major congenital and cardiac malformations.
Go to :

Materials and methods
1. Search strategy
A search of the literature from July 1998 to July 2015 was conducted using PubMed, Embase, Web of Science, and the Cochrane Library. The search terms included ‘citalopram’, ‘pregnancy’, ‘birth defects’, ‘congenital anomalies’, and ‘malformations’.
2. Inclusion and exclusion criteria
The inclusion criteria were as follows: the identified studies should be cohort or case-control studies in which exposure to citalopram occurred during pregnancy including the first trimester, and involved a control group of women unexposed to citalopram or other SSRIs. The papers were required to report on major malformations identified in the two groups. Major malformations were defined as the presence of serious or major structural defects that can have adverse effects on health or development. The classification followed EUROCAT (European Network for Surveillance of Congenital Anomalies) guidelines. The exclusion criteria were duplicate studies, reviews, meta-analyses, editorials/comments, case reports, experimental studies, trials published in languages other than English, studies with irrelevant outcomes or topics, studies describing breast milk exposure (not intrauterine exposure), studies related to the use of citalopram by non-pregnant adults, studies related to escitalopram use only (not citalopram), studies describing neonatal complications other than congenital anomalies, and studies that did not contain data relevant to the current work (
Fig. 1).
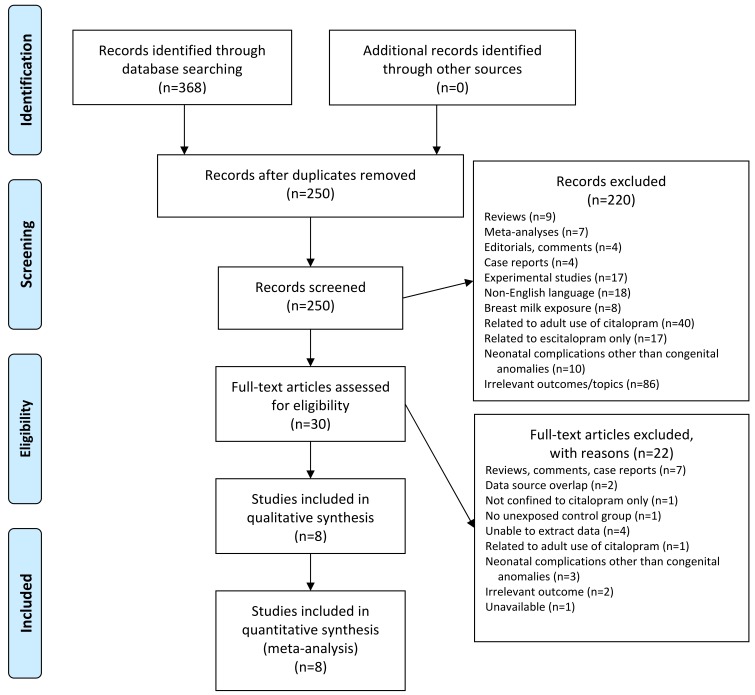 | Fig. 1Study selection process.
|
3. Study selection
Studies were selected in a staged manner. First, the titles and the abstracts of all screened articles were examined by two reviewers (HHK and SCH) independently. Second, the full text of each article was reviewed for all included articles. Third, if the suitability of the studies was doubtful, two other independent reviewers (AKH and LEH) discussed the study selection. For a reliable review process, we checked the quality of the reviewed papers using the systematic review appraisal sheet in Critical Appraisal Topic (
http://www.cebm.net/critical-appraisal/).
4. Data extraction
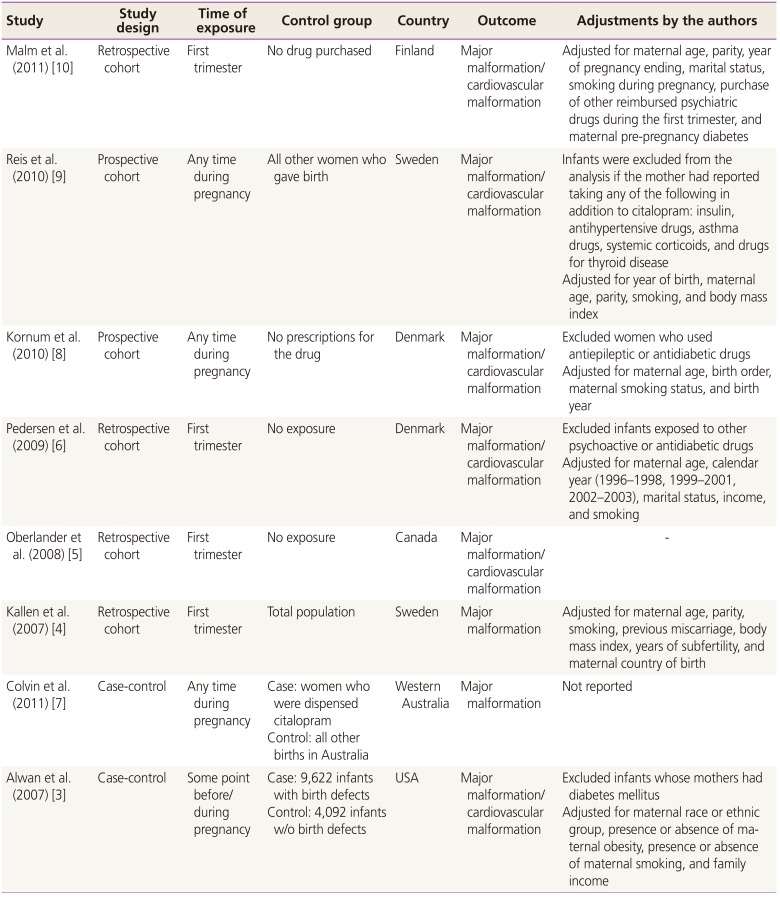
Data from the included studies were extracted and placed into 2×2 tables. Information of particular interest included data relating to study design, timing of exposure, control group, country, adjustments, size of the event group, and total number of participants. ORs, adjusted ORs, and/or 95% CIs were also included, if available. For case-control studies, adjusted ORs and 95% CIs were extracted without the specific number of participants.
5. Statistical analysis
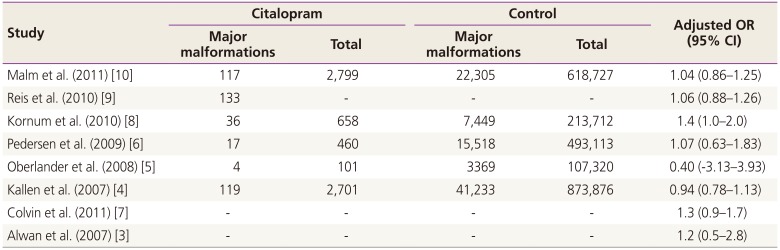
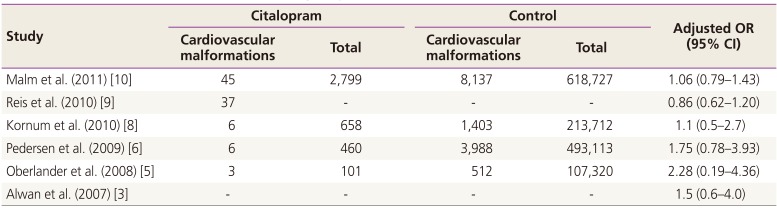
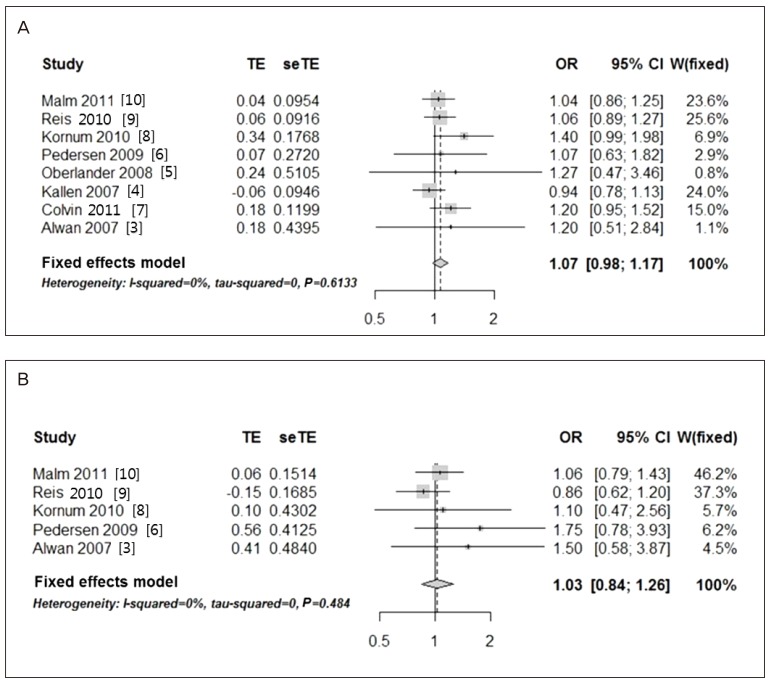
Heterogeneity tests revealed a low heterogeneity among the included studies; therefore, the fixed-effects model was used to obtain a summary OR based on the adjusted ORs extracted from individual studies. When all six studies reporting on the risk of cardiac malformations were tested, the heterogeneity was found to be too high to employ the fixed-effects model. A single outlier was identified by using a Galbraith plot, and this was excluded from the analysis. Finally, five studies were used for the evaluation of the risk of cardiac malformations. Higgins statistics and Q-statistics were calculated to test the heterogeneity of the studies. Additionally, Galbraith plots were used to check for heterogeneity and to identify outliers. Funnel plot analysis was used to detect publication bias. Although funnel plot asymmetry revealed a moderate degree of publication bias, application of the ‘trim and fill’ method did not result in substantial corrections, and the corresponding adjustments were not included in this study. The statistical analyses were conducted by using the R package ver. 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria).
Go to :

Discussion
To the best of our knowledge, this is the most updated meta-analysis to investigate the effect of in-utero exposure to citalopram on the risk of congenital anomalies. The results of the present study do not suggest an association between the use of citalopram during pregnancy and an increased risk of major or cardiac malformations.
The current data are consistent with the general conclusions of prior research and case reports, which suggested that citalopram use during pregnancy is not harmful. A recent systematic meta-analysis in 2013 also indicated that citalopram was not significantly associated with congenital malformations [
12]. In their analysis on several SSRI medications, unlike fluoxetine (OR, 1.14; 95% CI, 1.01 to 1.3) and paroxetine (OR, 1.29; 95% CI, 1.11 to 1.49), citalopram as analyzed in the current analysis did not increase risk of major malformations, including cardiac malformations. However, the meta-analysis by Myles et al. [
12] did not discuss the details of the included studies. Although they described seven and six studies included in the analysis for major and cardiac malformations, respectively, any information on study details could not be found in their text. When we compared their list of references with ours, three studies included in our analysis [
578] were absent in the study by Myles et al. [
12]. These three studies were potentially important to draw an updated conclusion in our analysis, because those study results all presented higher ORs in major malformations (ORs 1.2, 1.4, and 1.27 in Colvin et al. [
7], Kornum et al. [
8], and Oberlander et al. [
5], respectively) although none of the studies showed any statistical significance. Our study has an advantage in that it shows a larger amount of focused and specific information on citalopram as described in
Tables 1 to
3 [
345678910] compared with the study by Myles et al. [
12].
The importance of citalopram use in pregnancy has increased as it gains popularity with women of reproductive age. In a comparative analysis of 12 new-generation antidepressants, escitalopram and citalopram were ranked as highly acceptable [
13]. Moreover, citalopram is frequently used off-label for anxiety, panic disorder, dysthymia, premenstrual dysphoric disorder, body dysmorphic disorder, and obsessive-compulsive disorder [
141516]. The properties of citalopram, including its highly lipophilic nature, intermediate protein binding, and low molecular weight, enable it to cross the placenta. The minimal association with birth defects found in our meta-analysis may be explained by the low plasma levels of the drug during pregnancy. When women received a citalopram dose of 20 to 40 mg daily, the plasma concentrations of citalopram and metabolites were lower during pregnancy than those found in the non-pregnant state [
17]. Furthermore, neonatal plasma concentrations of citalopram and its metabolites at delivery were only 60% of the maternal plasma concentrations. It is suggested that the placental barrier minimizes the teratogenic effect of citalopram.
Serotonin has been suggested to be important in human development, especially cardiac and craniofacial developments [
181920]. Despite the safety concerns about the effects of SSRIs on human development
in utero, in general, these drugs are preferred over tricyclic antidepressants for the treatment of depression during pregnancy because they are known to be safer and have fewer adverse effects. Among SSRIs, citalopram is especially known to have few interactions in concomitant use with other drugs and is selects for 5-hydroxytryptamine uptake inhibition [
2122]. The trough plasma concentration of citalopram is even lower in pregnant women compared to non-pregnant women. This is likely a result from volume expansion and distribution and reduced plasma protein binding during pregnancy [
23]. The lower plasma concentration of citalopram during pregnancy and its selectivity imply a theoretically lower teratogenicity of this drug; however, some controversy about the association of citalopram with birth defects such as anencephaly, craniosynostosis, and omphalocele, as described by Alwan et al. [
3] should be resolved. Our results support the minimal or lack of association between citalopram use during pregnancy and birth defects, which is consistent with the results of Myles et al. [
12]. Notably, in contrast to citalopram, the safety of other SSRIs such as sertraline, fluoxetine, and paroxetine is still controversial, as these drugs have been consistently associated with the following birth defects: anencephaly and delayed ossification with sertraline; craniosysnostosis with fluoxetine; and anencephaly, right ventricular outflow tract obstruction defects, omphalocele, and gastroschisis with paroxetine [
312].
One limitation of the present meta-analysis stems from the unequal distribution of statistical weights, although our study included the most current up-to-date data. With respect to the risk of cardiac malformation, the weights of two of the studies far exceed that of the remaining studies combined. This gross disproportion may undermine the validity of the meta-analysis relating to the association with congenital cardiac malformations. An updated meta-analysis with extended data is still needed to overcome this limitation in the future. It is important to note that in addition to citalopram medication, depression itself would have negative impacts on teratogenicity before and during pregnancy. Women with depression are less likely to undergo pregnancy counseling and more likely to expose themselves to teratogens such as alcohol, smoking, and illicit drugs [
24]. However, it was practically difficult to differentiate the effect of depression from that of citalopram in our analyses.
When considering the results of the current study, it should be noted that all birth defects were categorized as major or cardiac malformations in our meta-analysis. With such a categorization, associations confined to a specific subset of birth defects may have been attenuated, and could be more difficult to detect. Thus, the weak overall association found in the present meta-analysis may be attributable, at least in part, to an excessive aggregation of defects. Nevertheless, considering the popular use of citalopram in women with psychological problems, including depression, and the ongoing concerns about the association between antenatal citalopram use and congenital anomalies, we are confident in using our data and results to counsel our patients and, at present, there is no evidence to consider citalopram as a teratogen for major or cardiac anomalies. In the future, more specific case categorization according to type of birth defect may result in outcomes that are more conclusive.
On the basis of our data, we cautiously suggest that physicians prescribe citalopram for use during pregnancy. The general principles relating to prescriptions during pregnancy should be applied; when prescribing citalopram, physicians should carefully weigh the benefits against the potential risk of congenital malformations, and counsel patients accordingly.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download