Abstract
Objective
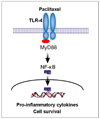
The objectives of this study was to evaluate the correlation between myeloid differentiation protein 88 (MyD88) expression and paclitaxel effects on epithelial ovarian cancer cells and to evaluate whether paclitaxel had anti-apoptotic signals.
Methods
Epithelial ovarian cancer cells isolated from ascites and established cell lines were treated with increasing concentrations of paclitaxel (0.2 to 20 µM) for 24 and 48 hours and cell viability was determined using the CellTiter 96 AQueous One Solution Cell Proliferation Assay. Cytokine profiling was performed from culture supernatants using the Luminex 200 system. Nuclear factor-kappaB (NF-κB) activity was determined using a Luciferase reporter system. Levels of phospho-extracellular signal-regulated kinase (p-ERK) were measured by Western blot analysis.
Results
A strong signal for MyD88 expression was observed in R182, 01-19b and SKOV3 cells (MyD88-positive). A2780, R454 and 01-28 cells showed low levels of MyD88 (MyD88-negative). Paclitaxel effectively decreased cell viability in MyD88-negative A2780, R454, 01-28 cells after 24 and 48 hours (57%, 49%, 42% and 35%, 28%, 29%, respectively). MyD88-positive cells were resistant to paclitaxel. There was a significant increase in caspase-3/7 activity following paclitaxel treatment in MyD88-negative cells. No significant change in caspase-3/7 activity was detected in MyD88-positive cells. Paclitaxel induced NF-κB activation and enhanced the secretion of interleukin-6 (IL-6) and IL-8 in a dose dependent manner and induced ERK phosphorylation on MyD88-positive cells.
Figures and Tables
 | Fig. 2Paclitaxel significantly decreases the number of viable MyD88-negative ovarian cancer cells (*P=0.000; General linear model repeated measures followed by Turkey method). The viability (in percentage, normalized to untreated cells) of MyD88-positive (R182, 01-19b, SKOV3) and -negative (A2780, R454, 01-28) cell lines after treatment with increasing concentrations of paclitaxel (0.2, 2, 20 µM) for 24 (A) and 48 (B) hours (P=0.000; General linear model repeated measures followed by Turkey method). |
 | Fig. 3Paclitaxel significantly induces apoptosis in MyD88-negative A2780 cells. Cells were treated with 2 µM paclitaxel for 24 hours. Activity of caspase-3/7 was measured using Caspase-Glo assay (*P=0.000; General linear model repeated measures followed by Turkey method). |
 | Fig. 4Differential effects of paclitaxel on nuclear factor-kappaB (NF-κB) activation in MyD88-positive R182 cells and -negative A2780 cells. Cells were transfected with a luciferase reporter plasmid activated by NF-κB and treated with 2 µM paclitaxel. NF-κB activity was measured as luminescence. Arrow indicates paclitaxel treatment. |
 | Fig. 5Cytokine profiles after paclitaxel treatment in MyD88-positive R182 cells. Cells were treated with paclitaxel (0.2, 2, 20 µM) for 48 hours and the levels of secreted cytokines were determined using Luminex 200 system (P=0.000; General linear model repeated measures followed by Turkey method). |
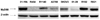
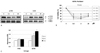 | Fig. 6(A) Effects of paclitaxel on extracellular signal-regulated kinase (ERK) activation in MyD88-positive and -negative cells. Cells were treated with paclitaxel (0.2, 2, 20 µM) for 24 hours and levels of phospho-ERK (p-ERK) and total ERK (t-ERK) were determined by Western blotting. (B) cell viability after paclitaxel at different times in MyD88-negative A2780 cells (*P=0.000; General linear model repeated measures followed by Turkey method). (C) In vitro growth pattern in cells with constitutive low ERK and high ERK. |
References
1. Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007. 82:751–770.

2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.

4. Okano J, Rustgi AK. Paclitaxel induces prolonged activation of the Ras/MEK/ERK pathway independently of activating the programmed cell death machinery. J Biol Chem. 2001. 276:19555–19564.

5. Lee M, Jeon YJ. Paclitaxel-induced immune suppression is associated with NF-kappaB activation via conventional PKC isotypes in lipopolysaccharide-stimulated 70Z/3 pre-B lymphocyte tumor cells. Mol Pharmacol. 2001. 59:248–253.
6. Taxman DJ, MacKeigan JP, Clements C, Bergstralh DT, Ting JP. Transcriptional profiling of targets for combination therapy of lung carcinoma with paclitaxel and mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor. Cancer Res. 2003. 63:5095–5104.
7. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. 454:436–444.

8. Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006. 66:3859–3868.

9. Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK). Semin Oncol. 2003. 30:105–116.

10. Zeng P, Wagoner HA, Pescovitz OH, Steinmetz R. RNA interference (RNAi) for extracellular signal-regulated kinase 1 (ERK1) alone is sufficient to suppress cell viability in ovarian cancer cells. Cancer Biol Ther. 2005. 4:961–967.

11. Hsu CY, Bristow R, Cha MS, Wang BG, Ho CL, Kurman RJ, et al. Characterization of active mitogen-activated protein kinase in ovarian serous carcinomas. Clin Cancer Res. 2004. 10:6432–6436.

12. Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll--the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008. 27:225–233.

13. Silasi DA, Alvero AB, Illuzzi J, Kelly M, Chen R, Fu HH, et al. MyD88 predicts chemoresistance to paclitaxel in epithelial ovarian cancer. Yale J Biol Med. 2006. 79:153–163.
14. Wang AC, Su QB, Wu FX, Zhang XL, Liu PS. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur J Clin Invest. 2009. 39:157–164.

15. Kim KH, Xie Y, Tytler EM, Woessner R, Mor G, Alvero AB. KSP inhibitor ARRY-520 as a substitute for Paclitaxel in Type I ovarian cancer cells. J Transl Med. 2009. 7:63.

18. Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998. 74:49–139.

19. Nakayama N, Nakayama K, Yeasmin S, Ishibashi M, Katagiri A, Iida K, et al. KRAS or BRAF mutation status is a useful predictor of sensitivity to MEK inhibition in ovarian cancer. Br J Cancer. 2008. 99:2020–2028.

20. Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999. 18:813–822.

21. Schmitz KJ, Wohlschlaeger J, Alakus H, Bohr J, Stauder MA, Worm K, et al. Activation of extracellular regulated kinases (ERK1/2) but not AKT predicts poor prognosis in colorectal carcinoma and is associated with k-ras mutations. Virchows Arch. 2007. 450:151–159.

22. Fan M, Chambers TC. Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist Updat. 2001. 4:253–267.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download