This article has been
cited by other articles in ScienceCentral.
Abstract
Anhidrotic ectodermal dysplasia (AED) is a rare hereditary disorder with a triad of sparse hair, dental hypoplasia, and anhidrosis. Here we report a case of AED with food allergy and atopic eczema. The patient was a 11-month-old boy admitted to our hospital with pyrexia for 2 weeks. He presented with a history of dry skin, eczema, and food allergy to egg. On clinical examination, his body temperature was 38.8°C, with dry skin and eczema almost all over the body, sparse eyebrows, and scalp hair. Laboratory investigations and physical examination did not show any evidence of infection. Radioallergosorbent test was positive to egg yolk, egg white, ovomucoid, milk, house dust, and house dust mite. As the child did not sweat despite the high fever, we performed the sweat test which revealed a total lack of sweat glands. Genetic examination revealed a mutation of the EDA gene and he was diagnosed as AED. His pyrexia improved upon cooling with ice and fan. His mother had lost 8 teeth and her sweat test demonstrated low sweating, suggestive of her being a carrier of AED. Atopy and immune deficiencies have been shown to have a higher prevalence in patients with AED. Disruption of the skin barrier in patients with AED make them more prone to allergic diseases such as atopic eczema, bronchial asthma, allergic rhinitis and food allergy. Careful assessment of the familial history is essential to differentiate AED when examining patients with pyrexia of unknown origin and comorbid allergic diseases.
Keywords: Anhidrotic ectodermal dysplasia, Pyrexia, Chronic eczema, Food allergy
INTRODUCTION
Anhidrotic ectodermal dysplasia (AED) is a rare hereditary disorder with triads of sparse hair, dental hypoplasia, and anhidrosis, proposed by Weech in 1929 [
1]. Patients with AED demonstrate abnormal faces, oligodontia, and dry and thin skin, hypoplasia of sebaceous glands leading to reduced ability to sweat, salivary glands, and mucous glands. Genetic and environmental factors in AED may contribute to disruption of the skin barrier making them more prone to allergic diseases such as atopic eczema, bronchial asthma, allergic rhinitis and food allergy, pneumonia and otitis media. Oligodontia results in poor oral intake, poor nutrition and reduced tears leading to corneal erosion. Most cases of AED are the X-linked form, while others are autosomal dominant form or autosomal recessive form.
Here we report a case of AED in a 11-month-old boy presenting with clinical manifestations of pyrexia, atopic eczema, and food allergy. The child was diagnosed as AED based on the sweat test and genetic analysis.
CASE REPORT
A 11-month-old boy was admitted to Nippon Medical School Musashi Kosugi Hospital with complaints of pyrexia for 2 weeks, poor oral ingestion and weight loss. He is the first child of nonconsanguineous parents. He was born by normal vaginal delivery at 38 weeks gestation with a birth weight of 2,282 g and birth height of 45 cm. He presented with a history of dry skin, chronic eczema, and egg allergy. He developed dry skin as early as 2 weeks of age and developed eczema at 1 month of age. Initially the eczema appeared on his face and neck and then extended to all over his body at 2 months of age. The eczema was treated with topical steroids. At 11 months of age (3 weeks prior to admission to our hospital), he developed hives on his face after eating cookies that contained egg and visited our hospital clinic. Based on the history of allergic symptoms on egg ingestion, clinical examination, and positive serum specific IgE to egg yolk (16.59 mg/dL), egg white (33.61 mg/dL), and ovomucoid (25.77 mg/dL), he was diagnosed as having egg allergy. On admission, he presented with extensive eczema all over the body with a SCORAD (SCORing Atopic Dermatitis) of 34.7, dry skin, sparse eyebrows and scalp hair, saddle nose, low-set ears, no teeth, and pigmentation around eyes (
Fig. 1A, B). He had polydipsia and polyuria. His height was 72.8 cm and body weight was 7.08 kg (Kaup index; 13.35), with a decrease in weight of about 500 g in 2 weeks. His vital signs on admission were as follows: consciousness clear; body temperature 38.8°C; blood pressure 94/52 mmHg; heart rate 148 beats/min; respiratory rate 26 breaths/min; oxygen saturation by pulse oximetry 99% (room air). He showed no signs of neck stiffness, cardiac murmur, nor abnormal breath sounds. Bacterial culture with blood, throat swab, stool, and urine showed no evidence of bacterial infection. The laboratory findings on admission are as described in
Table 1. White blood cell count was 17,470/μL with 21.9% neutrophils, C-reactive protein was <0.1 mg/dL, casual blood glucose level 110 mg/dL. Immunoglobulin G was 748 mg/dL, indicating that the patient does not suffer from hypogammaglobulinemia. The level of total serum IgE was 28 IU/mL and the radioallergosorbent test was positive to egg yolk 16.59 mg/dL (class 3); egg white 33.61 mg/dL (class 4); ovomucoid 25.77 mg/dL (class 4); milk 2.11 mg/dL (class 2); house dust 0.62 mg/dL (class 1); and house dust mite (
Dermatophagoides pteronyssinus) 0.56 mg/dL (class 1) (
Table 1). He was only sensitized to milk with no history of any allergic symptoms on milk ingestion. On admission, the child did not sweat despite the high fever. The sweat test [
2] revealed a total lack of sweat glands (
Fig. 1C, D). Radiograph of the mandible of patient showed a loss of teeth. Only bilateral canine teeth are observed (
Fig. 1F). Genetic examination was performed after acquiring informed consent from the parents and mutation of
EDA gene was observed (c.648_665 del). His pyrexia improved after cooling his body with ice and fan. Based on the findings of partial tooth dysplasia in his mother (she had lost 8 teeth), we performed her sweat test (
Fig. 1E). She was also suspected of having hypohidrosis, suggesting that she could be a carrier of AED.
Fig. 1
(A, B) Facial appearance of this case. Sparse eyebrows and scalp hair, saddle nose, low-set ears, pigmentation around eyes, dried skin, and thick rlips are observed. (C–E) Sweat test of cubital fossa: (C) The result of the patient shows no discoloration, indicating that there are no sweat glands. (D) The patient's mother showed partial presence of sweat gland. (E) Normal control. (F) Radiograph of the mandible of patient shows loss of teeth. Only bilateral canine teeth are observed (arrows).
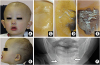
Table 1
Laboratory data on admission

|
Variable |
Value |
|
WBC |
17,470/µL |
|
RBC |
4,870,000/µL |
|
Hb |
9.2 mg/dL |
|
Ht |
30.9% |
|
PLT |
629,000/µL |
|
Neu |
21.9% |
|
Ly |
69.3% |
|
Mono |
4.8% |
|
Eosino |
3.6% |
|
Baso |
0.4% |
|
Glucose (casual) |
110 mg/dL |
|
AST |
43 IU/L |
|
ALT |
14 IU/L |
|
LDH |
369 IU/L |
|
CK |
63 IU/L |
|
Na |
134 mEq/L |
|
K |
4.7 mEq/L |
|
Cl |
102 mEq/L |
|
Ca |
9.7 mg/dL |
|
TP |
6.7 mg/dL |
|
ALB |
4.8 mg/dL |
|
BUN |
5.3 mg/dL |
|
CRE |
0.15 mg/dL |
|
CRP |
<0.1 mg/dL |
|
IgG |
748 mg/dL |
|
IgA |
26 mg/dL |
|
IgM |
66 mg/dL |
|
IgE (RIST) |
28 mg/dL |
|
RAST |
|
|
House dust |
0.62 mg/dL (class 1) |
|
Mites |
0.56 mg/dL (class 1) |
|
Egg yolk |
16.59 mg/dL (class 3) |
|
Egg white |
33.61 mg/dL (class 4) |
|
Ovomucoid |
25.77 mg/dL (class 4) |
|
Milk |
2.11 mg/dL (class 2) |
|
Urine |
|
|
Protein |
- |
|
OB |
- |
|
Glucose |
- |
|
Ketone |
- |
DISCUSSION
AED is a rare hereditary disorder with triads of sparse hair, dental hypoplasia, and anhidrosis. Patients with AED often suffer from heat accumulation and heatstroke because of anhidrosis. As a matter of fact, this patient was admitted to our hospital with complaints of pyrexia, which came from anhidrosis. The prognosis of AED is not so poor if the patients avoid heat accumulation. Immunodeficiency is sometimes known to coexist in patients with AED, therefore checking immunological function and bacterial culture would be necessary in examining AED patient with pyrexia.
Patients with AED often suffer from allergic diseases [
34]. According to the report of International Study of Asthma and Allergies in Childhood, the prevalence of allergic diseases with children of AED versus general population was high; eczema; 58.9% vs. 8.2%, bronchial asthma; 32.2% vs. 16.4%, allergic rhinitis; 76.1% vs. 38.9%. The prevalence of physician-diagnosed food allergies is 20.7%, which also exceeds known rates in the general population [
4]. The hypothesized mechanism is that a combination of genetic and environmental factors may contribute to disruption of the skin and mucosal barriers, permitting enhanced transmission and sensitization to irritants, allergens, and pathogens [
5].
Moreover, reduced mucous gland secretion in respiratory and gastrointestinal tracts, impaired salivary flow, and tearing can cause pneumonia, otitis media, atrophic rhinitis and corneal erosion. A multidisciplinary interventional approach for treatment involving the pediatrician, dermatologist, dentist, nutritionist, and genetic counselor should be the strategy to treat patients with AED.
In this case the patient presented with chronic eczema, food allergy, heat accumulation triggered by anhidrosis, and poor oral intake. He also had polydipsia and polyuria, for cooling and avoiding mucosal dryness.
The most common cause of AED is due to mutations in the
EDA gene (ectodysplasin A, Xq12), which occupies about 80% of AED. Other responsible genes are
EDAR (ectodysplasin A receptor, 2q12.3),
EDARADD (EDAR associated death domain, 1q43), and so on [
67]. EDA protein, a membrane protein which is expressed in keratinocytes, teeth and sweat glands stimulate nuclear factor-kappa B through EDAR and participate in the signaling of cells at the stage of ectodermal formation [
8]. In this case, a known mutation was observed [
9]. The relevant genes of the parents were not analyzed because we could not get the consent but based on the clinical findings in the mother, she was strongly suspected to be a patient of female type of AED. Generally, female career of X-linked disease does not present with symptoms of AED. But female careers of diseases such as Fabry disease, incontinentia pigmenti, and nephrogenic diabetes insipidus often show symptoms, which is less severe than that seen in males. These are explained by Lyonizaion, which hypothesizes random inactivation of X chromosome. One of the X chromosome in the female somatic cells are inactivated randomly during prenatal period. If most of the inactivated X chromosome is the mutated one, the phenotype of the individual is close to patient and if most of the inactivated X chromosome is normal, the phenotype becomes normal. The mother of this patient demonstrated dental hypoplasia and hypohydrosis. Moreover, the discoloration part of sweat test appears to be like Blaschko's lines. These observations suggest that the phenotype of the female career of X-linked AED may also present with symptoms, which is explained by Lyonization. In conclusion, we present here a case of AED with clinical manifestations of pyrexia, chronic eczema, and food allergy. The key educational and clinical point of this case is that a multidisciplinary approach is crucial in evaluating patients with AED. Moreover, it is important to assess the family history carefully to identify a diagnosis of AED when examining patients of unknown pyrexia with comorbid allergic diseases. Furthermore, genetic analysis and the phenotype of the patient's mother in this case suggest the availability of the hypothesis of Lyonization in AED which can be helpful with genetic counseling.
ACKNOWLEDGEMENTS
We thank Dr. Hajime Nakano, department of dermatology, Hirosaki University for the genetic analysis of this case.
References
1. Weech AA. Hereditary ectodermal dysplasia. Am J Dis Child. 1929; 37:766–790.
2. Wada M, Takagaki T. A simple and accurate method for detecting the secretion of sweat. Tohoku J Exp Med. 1948; 49:284.

5. Koguchi-Yoshioka H, Wataya-Kaneda M, Yutani M, Murota H, Nakano H, Sawamura D, Katayama I. Atopic diathesis in hypohidrotic/anhidrotic ectodermal dysplasia. Acta Derm Venereol. 2015; 95:476–479.


6. Wohlfart S, Hammersen J, Schneider H. Mutational spectrum in 101 patients with hypohidrotic ectodermal dysplasia and breakpoint mapping in independent cases of rare genomic rearrangements. J Hum Genet. 2016; 61:891–897.

7. Trzeciak WH, Koczorowski R. Molecular basis of hypohidrotic ectodermal dysplasia: an update. J Appl Genet. 2016; 57:51–61.

8. Kowalczyk-Quintas C, Schneider P. Ectodysplasin A (EDA) - EDA receptor signalling and its pharmacological modulation. Cytokine Growth Factor Rev. 2014; 25:195–203.


9. Zhang J, Han D, Song S, Wang Y, Zhao H, Pan S, Bai B, Feng H. Correlation between the phenotypes and genotypes of X-linked hypohidrotic ectodermal dysplasia and non-syndromic hypodontia caused by ectodysplasin-A mutations. Eur J Med Genet. 2011; 54:e377–e382.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download