This article has been
cited by other articles in ScienceCentral.
Abstract
Tipepidine hibenzate (Asverin) is commonly used as an antitussive drug for acute and chronic cough in various age groups and is generally safe and well-tolerated. However, we experienced a case of tipepidine hibenzate-induced anaphylactic shock in a 1-year-old boy. After ingesting cold medication including tipepidine hibenzate, the patient presented with generalized erythema and urticaria, swollen face, coughing, wheezing and vomiting, together with hypotension and a decreased level of consciousness. To identify the culprit drug, we performed skin prick tests (SPTs) and oral drug provocation tests (DPTs). SPTs revealed a negative reaction for all drugs, but DPTs caused a positive reaction only for a full therapeutic dose of tipepidine hibenzate. Physicians need to consider tipepidine hibezate as a culprit drug when anaphylaxis occurs after taking anticough or common cold medication.
Keywords: Anaphylaxis, Anaphylactic shock, Drug allergy, Asverin, Tipepidine hibenzate, Child
INTRODUCTION
Tipepidine hibenzate has been widely used as an antitussive drug since 1959 in Japan as well as in other Asian countries. This drug can sometimes be prescribed without any caution on the grounds that it is generally safe and well-tolerated with few adverse effects. We herein report a 1-year-old boy with anaphylactic shock induced by tipepidine hibenzate.
CASE REPORT
The patient was a 1-year-old boy with no history of allergy to any food or drugs. He was prescribed by his family doctor tipepidine hibenzate, pranlukast, carbocistein, amoxicillin/clavulanate, and tulobuterol for acute upper respiratory inflammation. Tipepidine hibenzate, pranlukast, and carbocistein had been taken before without any side effects, while amoxicillin/clavulanate and tulobuterol had never been taken until that moment. Thirty minutes after taking these 5 medications, the patient began to show whole-body itching and developed generalized erythema and urticaria, swollen face, coughing and wheezing, followed by a single instance of vomiting. He was brought to our hospital for the treatment of an allergic reaction.
At presentation in the emergency room, his vital signs were as follows: body temperature, 38.7°C; blood pressure, unmeasurable; pulse rate, 170 beats/min; respiratory rate, 48 breaths/min; and oxygen saturation 85%–89% on room air. In addition, he had a decreased level of consciousness (Glasgow Coma Scale 7). He was diagnosed with anaphylactic shock, and immediate treatment with an intramuscular injection of adrenaline (0.01 mg/kg) and intravenous d-chlorpheniramine (0.1 mg/kg) and hydrocortisone (10 mg/kg) was given. Because of insufficient improvement of his anaphylactic symptoms, he received an additional intramuscular injection of adrenaline (0.01 mg/kg), followed by a bolus injection of normal saline (10 mL/kg). His symptoms gradually disappeared after the treatment.
One or more of the above 5 drugs was strongly suspected of being the causative agent of his anaphylactic shock. An allergology workup was performed to clarify the identity of the culprit drug. First, skin prick tests (SPTs) with tipepidine hibenzate, pranlukast, carbocistein, amoxicillin/clavulanate, and tulobuterol were performed 3 weeks after the anaphylaxis event. A full therapeutic dose of these drugs was dissolved or suspended with 1.0 mL normal saline. A saline solution or the suspension of each drug and its 1:10 dilution were used for SPT. Histamine dihydrochloride (10 mg/mL) and normal saline were used as positive and negative controls, respectively. The SPT was considered positive if a wheal had a maximum diameter at least 3 mm greater than the negative control at 15 minutes [
1]. As shown in
Table 1, the results were negative for all drugs. We then performed an oral drug provocation test (DPT) 6 months after the anaphylaxis event. Each drug was administered in a 3-dose graded challenge according to a previously described method with minor modifications [
2]. This was conducted initially with 1/100th of the therapeutic dose, 1/10th of the therapeutic dose, and a full therapeutic dose, every hour. One hour after ingesting a full therapeutic dose of tipepidine hibenzate, the patient began to show overall itchiness. Subsequently, he developed erythema throughout the body and urticaria on his trunk (
Fig. 1). These symptoms promptly disappeared after treatment with oral ketotifen fumarate and betamethasone. In contrast, the provocation tests with the other drugs at all doses were negative (
Table 2). Based on the above results, we concluded that his previous episode of anaphylactic shock was attributable to tipepidine hibenzate. The patient was informed of an allergy to tipepidine hibenzate and advised not to take tipepidine hibenzate or any therapies including it again.
Table 1
Results of the skin prick tests

|
Variable |
Consentration (mg/mL) |
Wheal (mm × mm) |
|
Normal saline |
9*
|
0 × 0 |
|
Histamine dihydrochloride |
10 |
3 × 3 |
|
Tipepidine hibenzate |
0.8 |
0 × 0 |
|
8 |
0 × 0 |
|
Pranlukast |
3.5 |
0 × 0 |
|
35 |
0 × 0 |
|
Carbocistein |
30 |
0 × 0 |
|
300 |
0 × 0 |
|
Amoxiciillin/clavulanate |
60†
|
0 × 0 |
|
600†
|
0 × 0 |
|
Tulobuterol |
0.02 |
0 × 0 |
|
0.2 |
0 × 0 |
Fig. 1
The actual features of an oral drug provocation test with tipepidine hibenzate. Slight to moderate erythema is observed on the right axilla, chest, abdomen, and left inguina, along with some scratch wounds on the chest and abdomen. The inset is the high magnification of box. The yellow arrowheads indicate urticarial lesions.
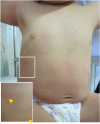
Table 2
Results of the oral drug provocation tests

|
Variable |
Dose (mg) |
Result |
|
Tipepidine hibenzate |
0.09 |
Negative |
|
0.9 |
Negative |
|
9 |
Positive |
|
Pranlukast |
0.48 |
Negative |
|
4.8 |
Negative |
|
48 |
Negative |
|
Carbocistein |
1.4 |
Negative |
|
14 |
Negative |
|
140 |
Negative |
|
Amoxiciillin/clavulanate |
10.1 |
Negative |
|
101 |
Negative |
|
1,010 |
Negative |
|
Tulobuterol |
0.0027 |
Negative |
|
0.027 |
Negative |
|
0.27 |
Negative |
DISCUSSION
Tipepidine hibenzate is a widely used and relatively safe nonopioid antitussive drug that acts centrally in the medulla oblongata by elevating the cough threshold [
3]. It is available with a prescription or in combination with other common cold remedies used for symptom relief. Unlike opioid antitussive drugs, such as codeine, it has no analgesic, euphoriant, or physical dependence-producing properties. Adverse reactions are infrequently reported and usually are not severe. Typical symptoms are a loss of appetite (1.1%) and constipation (0.5%) [
4]. To our knowledge, there have been only 2 adult cases of anaphylaxis caused by tipepidine hibenzate reported thus far [
56], and no pediatric cases have been described. This is the first paper describing severe adverse reactions to tipepidine hibenzate in a child that consequently led to anaphylactic shock.
In this patient, oral DPTs with tipepidine hibenzate resulted in overall itchiness with subsequent erythema throughout the body and urticarial rash on the trunk but did not trigger anaphylactic shock. This difference in response was probably due to the patient's physical condition, as he was in relatively poor health due to acute upper respiratory inflammation during the anaphylactic episode but was in a much better condition during the oral DPTs. In fact, it is generally known that cofactors, including exercise, ethanol, nonsteroidal anti-inflammatory drug, acute infections, stress, and perimenstrual status, can amplify anaphylaxis by decreasing the threshold of allergen exposure needed to trigger anaphylaxis and by amplifying the risk of anaphylaxis in patients with low or borderline allergen sensitization [
7].
In conclusion, we encountered a rare pediatric case of anaphylactic shock caused by tipepidine hibenzate that was confirmed by oral DPTs. Tipepidine hibenzate is commonly prescribed since it is generally regarded as safe, but clinicians need to keep in mind that tipepidine hibenzate can be a culprit drug when anaphylaxis occurs after taking anticough or common cold medication.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download