Abstract
Purpose
The purpose of this retrospective study was to evaluate clinical outcomes of endoscopic nerve decompression in patients with deep gluteal syndromes (DGS).
Materials and Methods
Between October 2013 and March 2015, 24 patients who underwent surgical treatment of DGS were retrospectively included in this study. The mean age was 47 years (range, 35 to 76 years), and there were 11 males and 13 females. The mean duration of pain was 12 months (range, 5 to 35 months) and the mean follow-up period was 32 months (range, 26 to 45 months). Clinical evaluations included the visual analog scale (VAS) pain score, modified Harris hip score (mHHS), and the symptom-rating scale.
Deep gluteal syndrome (DGS) is an underdiagnosed condition characterized by pain and/or dysesthesias in the buttock area, hip or posterior thigh and/or radicular pain due to a non-spinal sciatic nerve entrapment in the subgluteal space1). DGS-associated symptoms have been well described by several authors123) and clinical evaluations and physical examinations are critical for diagnosis as X-rays, magnetic resonance imaging (MRI) and magnetic resonance arthrography (MRA) are less effective in diagnosing DGS than in other hip pathologies45).
Imaging guided intra-articular and extra-articular injections (local anesthetic and steroids) have been described as helpful, precise and reproducible treatment options and at for discriminating complex pathologies6). Additionally, Park et al.4) reported the result of endoscopic sciatic nerve decompression in 60 patients with DGS. For patients with DGS who have moderate to severe symptoms that interfere with daily life or in whom conservative treatment is unsuccessful, sciatic nerve decompression through an open or endoscopic technique may be needed to reduce the pain137). It should be noted, however, that open sciatic nerve decompression carries a relatively high risk of postoperative complications (e.g., hematoma, infection, poor cosmetics, and long rehabilitation time) compared with endoscopic decompression48).
The purpose of this study is: (1) to evaluate clinical outcomes and complications after endoscopic nerve decompression in patients with DGS, and (2) to find clinical symptoms and physical examinations in our DGS patients.
Between October 2013 and March 2015, 24 patients with DGS (11 males, 13 females) received endoscopic sciatic nerve decompression and had at least 26 months of postoperative follow-up and were included in this retrospective analysis. The mean age, mean follow-up period and mean duration of symptoms was 47.12±12.60 years (range, 35–76 years), 32±6 months (range, 26–45 months) and 11.71±13 months (range, 5–35 months), respectively. The patients with spine and sacroiliac joint pathologies were excluded using MRI and physical examinations of the back and hip. Until the decision was made to pursue surgery, we followed the treatment protocol according Park et al.4) as described in Fig. 1.
Patient histories, clinical evaluations and physical examinations were used for clinical diagnosis of DGS.
We estimated the detailed clinical presentation and physical examinations according to Martin et al9).
Clinical presentations suggesting DGS included: 1) pain on walking10), 2) pain while sitting (especially in the ability to sit for >30 minutes), 3) radicular pain of the lower back or hip, 4) paresthesia, 5) night pain1), and 6) back pain1). The physical examinations included assessments of: 1) tenderness on sciatic notch, 2) the flexion-aduction-internal rotation (FADIR) test (positive when buttock pain aggravated), 3) Pace sign (pain and weakness with resisted abduction and external rotation of the hip11)), 4) Laségue test (pain with straight leg raise testing to 90° hip flexion12)), 5) seated piriformis test, and 6) hip range of motion (ROM) in a sitting position. The authors judged that each test was positive when pain was present. Patients with two or less positive clinical symptoms described above and two or less positive symptoms of the physical exam were diagnosed with DGS. Patients with recurrent DGS (i.e., those unsuccessfully treated with piriformis muscle injection more than three times) received endoscopic decompression.
MRA was performed in patients suspected of having an accompanying labral tear; MRI was performed in all others. Radiologic evaluations (i.e., pelvis anteroposterior plain radiography and MRA or MRI) were taken with patients in a supine position using a Picture Archiving and Communication System (PACS, Centricity Enterprise Web ver. 3.0; GE Medical Systems, Waukesha, WI, USA).
Endoscopic operations were performed with patients in a supine position on the hip arthroscopic table; a 70° hip arthroscope was used for visualization. Anterolateral and posterolateral portals were used for sciatic nerve exploration and an additional auxiliary posterolateral portal located at 3 cm posterior and 3 cm superior from the tip of the greater trochanter was used when needed (Fig. 2). Endoscopic examination of sciatic nerve was performed proximally from the quadratus femorsis muscle distally and beneath the proximal end of the linea aspera insertion of the gluteus maximus to the sciatic notch9).
Endoscopic sciatic nerve decompression included cautious removal of fibrovascular scar bands using a radiofrequency probe and release of the tendinous portion of the piriformis or obturator internus, or quadriceps femoris muscles compromised sciatic nerve excursion (Fig. 3). After decompression, the authors observed sufficient recovery of the sciatic nerve excursion on all hip motion of hip under endoscopy13). We also evaluated for the presence of sciatic variation using Beatons and Anson's classification and used an adhesion barrier bioresorbable membrane (ABBM; Guardix-SL® in 3 case and Guardix-SG® in 1 case; Genewel, Seongnam, Korea) to prevent re-adhesion in patients with severe adhesion.
From postoperative day 2, pain was tolerable in most patients, and partial weight bearing using crutches and all positions were permitted to help prevent re-adhesion. Clinical evaluations, physical assessments, and visual analog scale (VAS) and modified Harris hip score (mHHS) were re-evaluated after surgery to compare with presurgical results. As an additional postoperative assessment, the Benson and Schutzer classification8) (i.e., poor, fair, good, and excellent) was characterized (Table 1). An additional follow up was performed 6 months later and every year thereafter.
Statistical analyses were performed using SPSS (version 23.0; IBM Co., Armonk, NY, USA). Pre- and postoperative results were compared using paired t-tests. Continuous variables are indicated as means and standard deviations (i.e., preoperative and postoperative seated hip ROM). All statistical tests were 2-sided, and P-values <0.05 were considered statistically significant.
Clinical assessments and physical examinations are summarized in Table 2. Preoperatively, the most common clinical symptom was sitting pain (87.5%), followed by walking pain (the region of the sacroiliac joint, greater sciatic notch, and piriformis muscle that usually extends down the limb and causes difficulty with walking pain10), 50.0%) and radicular pain (relatively localized radicular pain to the posterior thigh, 41.7%). Postoperatively, these symptoms were significantly improved (P<0.001). Additionally, physical examination assessments (i.e., the seated piriformis test, tenderness on sciatic notch, Pace sign and seated hip ROM) show significant improvement following operations (P<0.001).
Sciatic nerve pathologies and abnormalities of the peritrochanteric space were not observed upon MRA; MRI assessments revealed: fibrous and fibrovascular bands (n=3), ganglion (n=2), Schwannoma (n=1) and nerve variation (n=1).
Sites of DGS in this study vary and are described in Table 3. As presented in Table 3, the DGS-causing locations vary and include: 1) fibrous and fibrovascular bands, 2) piriformis muscle and triceps coxae (the superior gemellus, obturator internus and inferior gemellus muscles), and 3) mass effect. In cases of mass effect, 2 were caused by ganglion compression of the sciatic nerve and one schwannoma. Successful endoscopic removal of both ganglion was achieved.
Operative results were unsatisfactory in two patients, one required revision of an open surgery for recurrence due to foreign body reaction to using Guardix-SG®, and the other had a schwannoma which could not be removed as it was located too superior to safely approach arthroscopically. Other than the single case of recurrence, no complications (e.g., infection or sciatic nerve palsy) were encountered.
The postoperative assessments of symptoms (i.e., rating scale described by Benson and Schutzer8)) were “good” or better in more than 87% of cases (Table 4). We assessed symptom improvement at postoperative 1 year. The mean VAS score for pain decreased from 7.1±0.9 to 2.5±1.5 (P<0.001). The mean mHHS increased from 59.4±6.5 to 85.0±8.3 (P<0.001), confirming significant symptom improvement after endoscopic decompression.
Although endoscopic sciatic nerve decompression may not be appropriate for all cases of DGS, there are various causes of DGS and it may be effective in certain cases. DGS involves pain in the buttocks caused by entrapment of the sciatic nerve by various adjacent muscles or scar tissue191415). Originating from the ventral rami of the L4 to S3, sciatic nerve roots maintain a single trunk in the pelvis and travel downward through the sciatic notch toward the piriformis muscle9). Deep gluteal space boundaries are: 1) anteriorly, femoral neck, 2) posteriorly, the gluteus maximus, 3) laterally, linea aspera of proximal femur, 4) medially, sacrotuberous ligament, 5) superiorly, inferior margin of the sciatic notch, and 6) inferiorly, hamstring muscle. Any structure in the gluteal space (e.g., piriformis muscle, fibrous bands, gluteal muscles, hamstring muscles, gemelli-obturator internus complexes, vascular bands) can induce sciatic nerve entrapment4).
Clinical manifestations of DGS are buttock pain and a complaint of the inability to sit for a long time16). DGS-associated pain can be similar to radiating pain of spine problems, thus making it difficult to diagnose. MRI scans of the spine can be used to exclude spine disease.
There are many of etiological factors associated with DGS (e.g., direct trauma of buttock or pelvis, hypertrophy of muscles in deep gluteal region, hematoma or neoplasm and anatomical variants the between piriformis and sciatic nerve41718)). As shown in our study, DGS can occur in various locations; although none of the cases in our study were caused by trauma, various distributions and nerve variations were present in one case which has been described as Type 2 according to Beatons and Anson's classification.
In our study, comprehensive physical examinations were performed. A seated piriformis test, tenderness on sciatic notch, Pace sign, seated hip ROM and FADIR test showed statistical significance pre- and post-operatively. As only four tests (i.e., seated piriformis test, tenderness on sciatic notch, Pace sign and seated hip ROM) were positive in over 50% of cases in our evaluation, these test may be useful methods not only for estimating preoperative diagnosis but also to assess postoperative results. In FADIR test of all cases of combined femoroacetabular impingement (FAI) (6 cases), however, preoperative positive and postoperative negative results were observed. Therefore, despite statistical significance, we cannot conclude that this test is useful for diagnosing DGS.
When caused by trauma or over-use, some authors suggested that MRI is required to detect complications, inflammatory changes or the formation of fibrous bands in which case infiltration or endoscopic debridement of the sciatic nerve may be required. However, all our cases were non-traumatic and MRI or MRA were performed in all 24 cases. MRA was performed in patients with possible labral tears, while MRI was performed in others. Martin et al.9) reported that MRA cannot detect sciatic nerve pathologies despite the trial of several technique because of limitation of reveal of the peritrochanteric space. So they suggested the bilateral dynamic electromyogram (EMG) test for management of DGS. While we agree with this recommendation, we were unable to consult a neurologist in this study, thereby limiting our evaluation.
Open decompression for DGS can resolve clinical symptoms (e.g., sensory symptoms, tenderness and or paresthesia, foot drop etc19)); however, there are risks associated with this open technique (e.g., hematoma, infection, cosmetic problems and long duration of rehabilitation in open decompression420)). In our study, none of these common complications were observed, so we suggest that endoscopic decompression has fewer risks than open decompression. However, endoscopic decompression failed in several cases in this study. One patient had a schwannoma which was difficult to safely manage endoscopically. Additionally, one case was situated as re-adhesion due to ABBM. A foreign body reaction due to the Guardix-SG®-an ABBM consisting of alginate and poloxamer-formed and extensive adhesions occurred around the sciatic nerve. Among the 24 cases included in this study, we used ABBM (Guardix-SL® in 3 case and Guardix-SG® in 1 case) to prevent re-adhesion in patients with severe adhesion; only one case had a foreign body reaction as previously described. Adhesion due to this foreign body was very severe and was not released using the endoscopic procedure. We revised to an open surgery and achieved satisfactory release (Fig. 4). Histopathological examination revealed benign-looking fibroadipose tissue with foreign body giant cells. Considering these two cases, conventional open surgery may be recommended for adjust decompression and avoiding neurologic complications of revision operations. Also, if the main lesion is the sciatic nerve itself (e.g., schwannoma), it should be managed openly and more delicately to avoid neurologic complications.
This study has the following limitations, 1) small sample size, 2) large number of DGS patients (23%) who had FAI treated at same time, 3) no single method validated for the diagnosis of DGS, and 4) not including emerging diagnosis tools such as dynamic EMG testing. Further studies should be conducted that include dynamic testing such as bilateral dynamic EMG testing and more patients who are excluded such as FAI and other diseases.
Endoscopic sciatic nerve decompression is a useful procedure to improve hip function and reduce DGS-associated pain. There are no specific radiological findings (e.g., simple radiography and MRI/MRA) to diagnose DGS, therefore clinical presentations and physical examinations are critical for accurate diagnosis. The authors suggest that pain while sitting, tenderness on sciatic notch, Pace test and seated piriformis tests are closely related to a diagnosis of DGS. Additionally, the endoscopic surgical approach described herein appears to be effective at reducing postoperative complications and improving clinical symptoms associated with DGS.
References
1. Hernando MF, Cerezal L, Pérez-Carro L, Abascal F, Canga A. Deep gluteal syndrome: anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015; 44:919–934. PMID: 25739706.

2. Martin HD, Reddy M, Gómez-Hoyos J. Deep gluteal syndrome. J Hip Preserv Surg. 2015; 2:99–107. PMID: 27011826.

3. Hopayian K, Song F, Riera R, Sambandan S. The clinical features of the piriformis syndrome: a systematic review. Eur Spine J. 2010; 19:2095–2109. PMID: 20596735.

4. Park MS, Yoon SJ, Jung SY, Kim SH. Clinical results of endoscopic sciatic nerve decompression for deep gluteal syndrome: mean 2-year follow-up. BMC Musculoskelet Disord. 2016; 17:218. PMID: 27206482.

5. Martin HD, Kivlan BR, Palmer IJ, Martin RL. Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region. Knee Surg Sports Traumatol Arthrosc. 2014; 22:882–888. PMID: 24217716.

6. Filler AG, Haynes J, Jordan SE, et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005; 2:99–115. PMID: 15739520.

7. Boyajian-O'Neill LA, McClain RL, Coleman MK, Thomas PP. Diagnosis and management of piriformis syndrome: an osteopathic approach. J Am Osteopath Assoc. 2008; 108:657–664. PMID: 19011229.
8. Benson ER, Schutzer SF. Posttraumatic piriformis syndrome: diagnosis and results of operative treatment. J Bone Joint Surg Am. 1999; 81:941–949. PMID: 10428125.
9. Martin HD, Shears SA, Johnson JC, Smathers AM, Palmer IJ. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy. 2011; 27:172–181. PMID: 21071168.

10. Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am. 2004; 35:65–71. PMID: 15062719.

11. Pace JB, Nagle D. Piriform syndrome. West J Med. 1976; 124:435–439. PMID: 132772.
12. Freiberg AH. Sciatic pain and its relief by operations on muscle and fascia. Arch Surg. 1937; 34:337–350.

13. Coppieters MW, Alshami AM, Babri AS, Souvlis T, Kippers V, Hodges PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res. 2006; 24:1883–1889. PMID: 16838375.

14. Cox JM, Bakkum BW. Possible generators of retrotrochanteric gluteal and thigh pain: the gemelli-obturator internus complex. J Manipulative Physiol Ther. 2005; 28:534–538. PMID: 16182029.

15. Meknas K, Christensen A, Johansen O. The internal obturator muscle may cause sciatic pain. Pain. 2003; 104:375–380. PMID: 12855348.

16. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy. 2000; 16:578–587. PMID: 10976117.

17. Beaton LE, Anson BJ. The sciatic nerve and the piriformis muscle: their interrelation a possible cause of coccygodynia. J Bone Joint Surg Am. 1938; 20:686–688.
18. Kitagawa Y, Yokoyama M, Tamai K, Takai S. Chronic expanding hematoma extending over multiple gluteal muscles associated with piriformis syndrome. J Nippon Med Sch. 2012; 79:478–483. PMID: 23291848.

19. Issack PS, Kreshak J, Klinger CE, Toro JB, Buly RL, Helfet DL. Sciatic nerve release following fracture or reconstructive surgery of the acetabulum. Surgical technique. J Bone Joint Surg Am. 2008; 90(Suppl 2):227–237. PMID: 18829936.
20. Beauchesne RP, Schutzer SF. Myositis ossificans of the piriformis muscle: an unusual cause of piriformis syndrome. A case report. J Bone Joint Surg Am. 1997; 79:906–910. PMID: 9199390.
Fig. 1
Flow-chart showing treatment process. A flow-chart showing the decision making process for endoscopic sciatic nerve decompression of seating posterior hip pain.
Adapted from the article of Park MS et al. (BMC Musculoskelet Disord 2016;17:218)4).
Fig. 2
Endoscopic portals placement including additional auxiliary portal. (A) Anterolateral portal, (B) posterolateral portal, (C) auxiliary posterolateral portal. Allowed for better visualization of the sciatic nerve up to the sciatic notch.
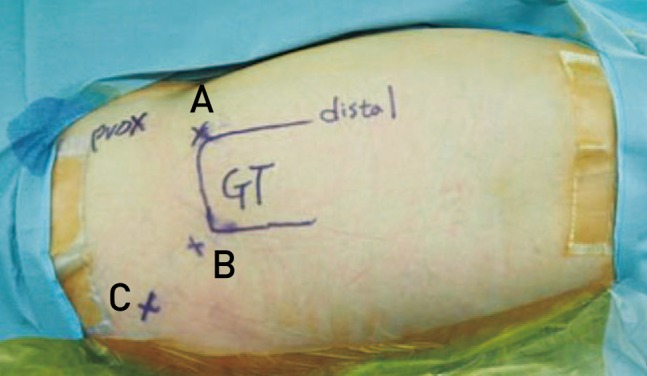
Fig. 3
(A) Entrapment of sciatic nerve (SN) by fibrovascular band (FVB) and piriformis muscle (PM) were observed. (B) SN after decompression by releasing FVB and PM were observed. (C, D) Adequate excursion by probing after decompression were observed.
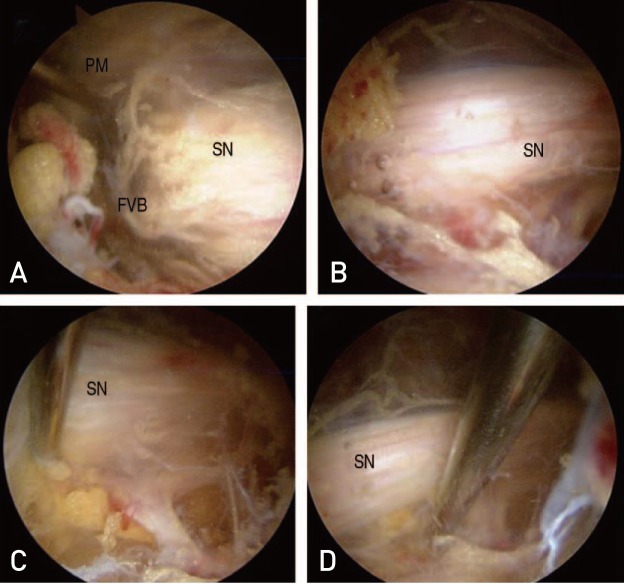
Fig. 4
(A–C) Sciatic nerve (SN) is compressed by fibrous tissue (FT), inferior gluteal vessels (IGV) and priformis muscle (PM). (D) After decompression of SN (also used Guardix-SG for avoiding recurrence which is an adhesion barrier bioresorbable membrane).
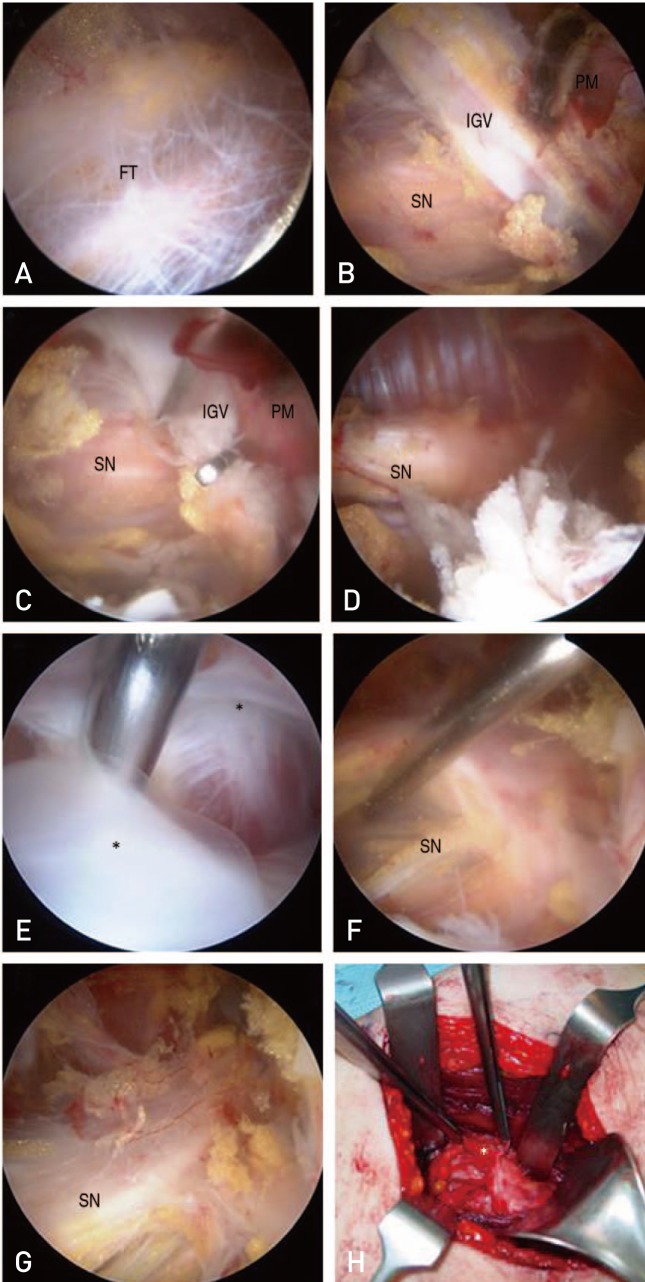
Table 1
The Symptom-Rating Scale by Benson and Schutzer8)

Data from the article of Benson ER and Schutzer SF (J Bone Joint Surg Am 1999;81:941–949).8)
Table 2
Clinical Symptoms and Physical Examinations (n=24)
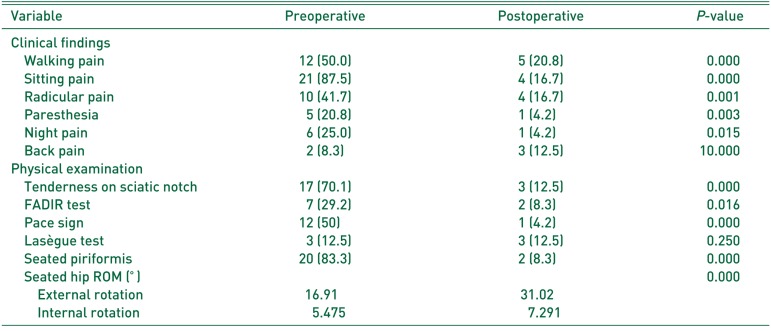
Table 3
Causes of Sciatic Nerve Entrapment (n=24)

| Compromising structure | Number |
|---|---|
| Fibrous and fibrovascular bands | 13 |
| Piriformis muscle and triceps coxae | 8 |
| Mass effect | |
| Ganglion | 2 |
| Schwannoma | 1 |
| Total | 24 |
Table 4
The Symptom-rating Scale by Benson and Schutzer8) in This Study

| Surgical outcome rating | Data |
|---|---|
| Excellent | 15 (62.5) |
| Good | 6 (25.0) |
| Fair | 1 (4.0) |
| Poor | 2 (8.3) |
| Total | 24 (100) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download