1. Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010; 34:609–619.

2. Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014; 46:153–157.

3. Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, et al. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): a review of endoscopic and clinicopathological features. World J Gastroenterol. 2016; 22:10523–10531.

4. Sato Y, Fujino T, Kasagawa A, Morita R, Ozawa SI, Matsuo Y, et al. Twelve-year natural history of a gastric adenocarcinoma of fundic gland type. Clin J Gastroenterol. 2016; 9:345–351.

5. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 15th ed. Tokyo: Kanehara Shuppan;2017.
6. Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013; 63:318–325.

7. Hidaka Y, Mitomi H, Saito T, Takahashi M, Lee SY, Matsumoto K, et al. Alteration in the Wnt/β-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum Pathol. 2013; 44:2438–2448.

8. Lewin E, Daroca P, Sikka S, Wu T, Nakanishi Y. Gastric adenocarcinoma of the fundic gland type treated by endoscopic mucosal resection: a case report and review of the literature. Case Rep Pathol. 2016; 2016:8646927.

9. Kusumoto C, Shigehara K, Take S, Ishiki K, Taniguchi K. Slow progression of gastric adenocarcinoma of fundic gland type: a case report. Nippon Shokakibyo Gakkai Zasshi. 2016; 113:2042–2049.
10. Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, et al. Gastric adenocarcinoma of fundic gland type: five cases treated with endoscopic resection. World J Gastroenterol. 2015; 21:8208–8214.

11. Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (gastric adenocarcinoma of fundic gland type). Int J Clin Exp Pathol. 2011; 4:797–798.
12. Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig Endosc. 2014; 26:293–294.

13. Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, et al. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997; 74:112–121.

14. Tanabe H, Iwashita A, Ikeda K, Ohta A, Yao K. Histopathological characteristics of gastric adenocarcinoma of fundic gland type. Stomach Intest. 2015; 50:1469–1479.
15. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
16. Chiba T, Kato K, Masuda T, Ohara S, Iwama N, Shimada T, et al. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc. 2016; 28:722–730.

17. Yao T, Ueyama H, Kushima R, Nakajima Y, Hirakawa K, Oshiro Y, et al. New type of gastric carcinoma-adenocarcinoma of the fundic gland type: its clinicopathological features and tumor development. Stomach Intest. 2010; 45:1192–1202.
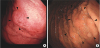

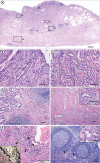





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download