Abstract
Purpose
Composite neuroendocrine-exocrine carcinomas are malignancies that have two distinct components residing within the same tumor: an adenocarcinomatous portion and a neuroendocrine portion. This is rare in gastric cancers; however, poorly differentiated adenocarcinomas can sometimes reveal evidence of neuroendocrine features (NEF) or be 'mixed endocrine and exocrine carcinomas'. This study aimed to review NEF in gastric adenocarcinoma and to evaluate its prognostic significance.
Materials and Methods
We selected 29 patients who were diagnosed with gastric adenocarcinoma with NEF and received gastrectomies at the Department of Surgery, Ajou University Hospital between January 2001 and December 2009. We analyzed the clinicopathologic features of gastric cancer with NEF and the prognosis associated with such tumors.
Results
The pathologic result with respect to TNM staging of the gastric cancers with NEF were as follows: 5 cases of T1, 5 cases of T2, 10 cases of T3, and 9 cases of T4. There were 7 cases of N0, 7 cases of N1, 8 cases of N2 and 7 cases of N3. The staging of patients with NEF was higher than that of patients without NEF. Especially tumor lymphovascular invasion rate was 82.8%. The overall survival of patients with gastric cancer characterized by NEF was 73.8 months.
Neuroendocrine tumors originating from diffuse neuroendocrine cells are only partially classified under the same scheme, while in part follows the classification of the tumors of the specific site of origin (i.e., in the respiratory and urogenital tract); such almost contradictory state of affairs concerns also the group of mixed neuroendocrine and non-neuroendocrine neoplasms.(1) Neuroendocrine tumors may give rise to pure endocrine tumors or neoplasms that have aspects of both neuroendocrine differentiation and non-neuroendocrine features.(2)
Gastric epithelial tumors that are composed of exocrine cells and neuroendocrine cells can be divided into two broad groups: pure endocrine tumors, i.e., adenomas or adenocarcinomas with interspersed neuroendocrine cells, and typical endocrine tumors, i.e., mixed exocrine-neuroendocrine tumors in which neuroendocrine components represent at least one-third to half of the tumor tissue.(2-4) Both 'pure or typical' carcinomas are rare in gastric cancer. However, sometimes gastric adenocarcinomas show evidence of 'neuroendocrine features (NEF)' or act as 'mixed endocrine and exocrine carcinomas'.(1)
Histological differentiation of gastric cancer has long been accepted as one of the indicators of prognosis. More specifically, the degree of tumor cell differentiation correlates with neoplasm aggressiveness.(5-8) However, there have been few studies concerning the correlation between NEF and disease progression.
This study aimed to review NEF in gastric adenocarcinoma and to evaluate the prognostic significance of this diagnosis.
We selected 29 patients who were diagnosed gastric adenocarcinoma with NEF and who received a gastrectomy at the Department of Surgery, Ajou University Hospital between January 2001 and December 2009. We retrospectively collected data using medical records and telephone interviews. We analyzed patient's age, gender, tumor location, gross findings, tumor size, cancer stage, pathologic classification (i.e., Lauren classification, lymphatic or vascular invasion) and overall survival.
All tumors were fixed with 10% formalin and embedded in paraffin immediately after resection. They were then stained with hematoxylin and eosin and evaluated with respect to the histological classification of the lesion. When the tumor consisted of both neuroendocrine and adenocarcinomatous components juxtaposed within the individual tumor and the neuroendocrine components occupied at least 30% of the tumor tissue, we diagnosed NEF in the adenocarcinoma. Following this, we performed additional immunohistochemistry of the NEF tumor. NEF stained a particular color when stained with chromogranin A or synaptophysin.
There were 23 men and 6 women who were enrolled in the study with NEF of the gastric adenocarcinoma. The mean age of the sample was 58 years old (range: 30 to 80 years old). The tumor size ranged from 2.0 to 12.0 cm in maximal diameter, with a median of 4.5 cm. Two of the tumors were situated in the upper third; six tumors were situated in the middle third; and twenty-one were situated in the lower third. According to the Borrmann gross type classification, there was 1 Borrmann type I case, 8 Borrmann type II cases, 14 Borrmann type III cases, 1 Borrmann type IV case and 5 early gastric cancer cases. The pathologic result of gastric cancer with NEF was classified using the tumor-nod-metastasis (TNM) staging system. There were 5 T1 cases, 5 T2 cases, 10 T3 cases, 8 T4a cases, and 1 T4b case. There were 7 N0 cases, 7 N1 cases, 8 N2 cases, 2 N3a cases and 5 N3b cases. Finally, there were 4 stage I cases (13.8%), 12 stage II cases (41.4%), and 13 stage III cases (44.8%) (Table 1).
On histological examination, lymphatic invasion was evident in 24 cases (82.8%), vascular invasion was evident in 17 cases (58.6%) and neural invasion was evident in 11 cases (37.9%). By Lauren classification, there were 12 diffuse-type cases (41.4%), 13 intestinal-type cases (44.8%), 4 mixed-type cases (13.8%) (Table 2).
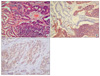
After we diagnosed NEF, sometimes we subsequently performed additional Chromogranin A and synaptophysin immunohistochemical staining. Chromogranin A was performed on 16 cases, and 10 tumors (62.5%) were positive for chromogranin A. Synaptophysin was performed on 17 cases, and 13 tumors (76.5%) were positive for synaptophysin (Fig. 1).
All patients were treated using gastrectomy with lymph node dissection adopted by adenocarcinoma in gastric cancer. If the final pathologic stage of the tumor was greater than stage II, we performed adjuvant chemotherapy. No patients had post-operative mortality or critical complications. The median follow-up length was 22 months (range: 3 to 108 months), and the mean survival time was 73.8 months (Fig. 2).
According to the '2009 Nationwide survey on surgically treated gastric cancer patients in South Korea', the proportion of nodal metastasis was 37.4%.(9) However, we found that a higher proportion of tumors with NEF showed lymph node metastasis (75.9%) and lymphatic invasion (82.8%). We compared these data with another study data of 'Helicobacter pylori related gastric cancer' which received gastrectomy at our institute between January 2006 and December 2006. These result also showed NEF had more lymphatic or vascular invasion (Table 2). However theses data had limitation, which was difference of the time period between two groups. Kim et al.(10) suggest that there is a paracrine effect of tumor growth factor that is caused by neuroendocrine tumors. Eren et al.(11) also suggest that neuroendocrine cells may contribute to angiogenesis by expressing VEGF, especially in advanced stage cases. We noticed that there were more advanced stage tumors among the NEF group than the non-NEF group.(9) This finding was also observed in the study by Ooi et al.(4) for gastric carcinomas, Tamura et al.(12) for endometrial adenocarcinomas and Allen et al.(13) for prostatic adenocarcinomas. Similar to these studies, we think that this is likely due to the expression of multi potent stem cells, which are more common in advanced stage tumors.
In our study, their mean survival time was 73.8 months and seven (24%) of them had died of disease within 2 years. Generally, the survival time was strongly correlated with tumor stage, however we did not have sufficient NEF data for individual stages to compare survival time between NEF and non-NEF tumors.
We used two neuroendocrine markers, chromogranin A and synaptophysin. Chromogranin A is widely distributed in the secretary granules of most polypeptide-producing endocrine tissues and is considered to be very useful as a diagnostic aid for normal neuroendocrine and tumor cells.(14) In our study, 62.5% of the NEF showed immunoreactivity for chromogranin A. Synaptophysin is an integral membrane glycoprotein that was originally isolated from bovine neuronal presynaptic vesicles and is considered to be a significant neuroendocrine marker.(15) In our study, 76.5% of NEF were positive for synaptophysin. Therefore, both chromogranin A and synaptophysin have been shown to be valuable markers for detecting neuroendocrine cells. However, we did not perform this immunohistochemical stain for all tumor cells. Only 29 tumors had the exact diagnosis of NEF, although we performed more than two thousand gastrectomies. Eren et al.(11) found neuroendocrine differentiation in tumor cells among 45% of the conventional gastric adenocarcinomas. Waldum et al.(16) suggested a correlation between diffuse-type gastric carcinomas and those with neuroendocrine differentiation. Compare with our another data (H. pylori related gastric cancer), NEF and non-NEF showed similar Lauren classification (Table 2).
In conclusion, NEF was more frequent in advanced stage gastric adenocarcinomas and was more commonly associated with lymph node metastasis. If preoperative biopsy show the presence of NEF in gastric adenocarcinomas should thus raise concern for lymph node metastasis, and a lymph node dissection should be completed in such patients.
Figures and Tables
Fig. 1
Histological finding of H&E stain and immunohistochemistry. (A) Adenocarcinoma with neuroendocrine features (H&E stain, ×200). (B) Positive in area of neuroendocrine tumor (Gold color) (Synaptophysin stain, ×200). (C) Positive in area of neuroendocrine tumor (Gold color) (Chromogranin A stain, ×200).
References
1. Volante M, Righi L, Asioli S, Bussolati G, Papotti M. Goblet cell carcinoids and other mixed neuroendocrine/nonneuroendocrine neoplasms. Virchows Arch. 2007. 451:Suppl 1. S61–S69.

2. Rindi G, Arnold R, Bosman FT. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. WHO classification of tumors of the digestive system. 2010. Lyon: IARC.

3. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987. 11:Suppl 1. 71–86.

4. Ooi A, Hayashi H, Katsuda S, Nakanishi I. Gastric carcinoma cells with endocrine differentiation show no evidence of proliferation. Hum Pathol. 1992. 23:736–741.

5. Park JM, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ, et al. Gastric cancer histology: clinicopathologic characteristics and prognostic value. J Surg Oncol. 2008. 98:520–525.

6. Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993. 218:583–592.

7. Sim YK, Kim CY, Jeong YJ, Kim JH, Hwang Y, Yang DH. Changes of the clinicopathological characteristics and survival rates of gastric cancer with gastrectomy: 1990s vs early 2000s. J Korean Gastric Cancer Assoc. 2009. 9:200–206.

8. Seo WH, Seo BJ, Yu HJ, Lee WY, Lee HK. Analysis of prognostic factors in 1,435 surgically treated patients with gastric cancer. J Korean Gastric Cancer Assoc. 2009. 9:143–151.

9. Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011. 11:69–77.

10. Kim KM, Kim MJ, Cho BK, Choi SW, Rhyu MG. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002. 440:85–93.

11. Eren F, Celikel C, Güllüoğlu B. Neuroendocrine differentiation in gastric adenocarcinomas; correlation with tumor stage and expression of VEGF and p53. Pathol Oncol Res. 2004. 10:47–51.
12. Tamura T, Jobo T, Watanabe J, Kanai T, Kuramoto H. Neuroendocrine features in poorly differentiated endometrioid adenocarcinomas of the endometrium. Int J Gynecol Cancer. 2006. 16:821–826.
13. Allen FJ, Van Velden DJ, Heyns CF. Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol. 1995. 75:751–754.
14. Wilson BS, Lloyd RV. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984. 115:458–468.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download