Abstract
Laparoscopic distal gastrectomy has become widespread as a treatment for early gastric cancer in eastern Asia, but a standard method for setting the stomach transection line has not been established. Here we report a novel method of setting this line based on anatomical landmarks. At the start of the operation, two anatomical landmarks along the greater curvature of the stomach were marked with ink: the proximal landmark at the avascular area between the last branch of the short gastric artery and the first branch of the left gastroepiploic artery, and the distal landmark at the point of communication between the right and left gastroepiploic arteries. Just before specimen retrieval, the stomach was transected from the center of these two landmarks toward the lesser curvature. Then, about two-third of the stomach was reproducibly resected, and gastroduodenostomy was successfully performed in 26 consecutive cases. This novel method could be used as a standard technique for setting the transection line in laparoscopic distal gastrectomy.
Laparoscopic distal gastrectomy (LDG) has been gaining popularity as a treatment option for stomach pathologies, particularly for early gastric cancer in eastern Asia. Standard procedures for lymph node dissection1 and reconstruction2 in LDG have been established. LDG is the operation of choice for gastric cancers in the distal half of the stomach, and resection of more than two-third of the stomach with adequate lymph node dissection is recommended by the Japanese gastric cancer treatment guidelines.3 However, it can be difficult to estimate the size of the stomach during the laparoscopic operation and choose the most appropriate resection line without reliable anatomical landmarks. In this report, we present a novel and straightforward method for choosing the location of the transection line based on anatomical landmarks.
A total of 51 patients diagnosed with gastric adenocarcinoma confined to the distal half of the stomach underwent LDG in Toyooka Hospital between April 2013 and November 2014. Preoperative diagnostic evaluations included endoscopy, an upper gastrointestinal series, and computed tomography. In patients with cancer in the middle body of the stomach, preoperative endoscopic marking of the tumor with tattooing was performed. Among these patients, Billroth II or Roux-en-Y reconstruction was performed in those in whom the extent of LDG resulted in a small remnant stomach because of the tumor location. Billroth-II reconstruction was carried out in 17 elderly patients over 75 years of age, and the Roux-en-Y reconstruction in 8 younger patients or in those with a hiatal hernia. The remaining 26 patients underwent LDG followed by gastroduodenostomy.
Patients were placed under general anesthesia in the supine, reverse Trendelenburg position with their legs apart. After pneumoperitoneum was established using the Hasson technique at the umbilicus, a flexible electro-laparoscope was introduced through the umbilical trocar. Four other operating trocars were placed in the upper abdomen as previously described.1,2,6
At the start of the operation, two anatomical landmarks on the greater curvature of the stomach were recognized and marked with Gentian violet ink (Fig. 1, 2).7 The first point, the proximal landmark, was the avascular area between the last branch of the short gastric artery (SGA) and the first branch of the left gastroepiploic artery (Fig. 2A). The other point, the distal landmark, was the point of communication between the right gastroepiploic arteries (RGEA) and left gastroepiploic arteries (LGEA) (Fig. 2B). Subsequently, lymph node dissection was performed along the greater curvature (No. 4sb, 4d, 6), and after duodenal transection, the suprapancreatic lymph node dissection and that along the ascending branch of the left gastric artery (LGA) (No. 1) were performed. When an adequate negative surgical margin along the lesser curvature was achieved, a 3-cm line distal to the esophagogastric junction was chosen as the transection point along the lesser curvature. When there was potential for a positive margin, an additional 1- to 2-cm tissue proximal to the margin was removed. On the greater curvature, the transection point was set at the center of the 2 marked points. The transection line was outlined with Gentian violet ink (Fig. 2D), and then transected with endoscopic linear staplers (Fig. 1). The proximal margin of the specimen was sent for intraoperative frozen section analysis when necessary.
All patients underwent LDG with gastroduodenostomy as planned. The clinicopathological characteristics of the patients are summarized in Table 1. No intraoperative complications or conversions to open surgery occurred. Pathological tumor staging was as follows: Stage IA, 12 cases; Stage IB, 5 cases; and Stages IIA, IIB, and IIIA, 3 cases each (Table 1). Short-term surgical outcomes are summarized in Table 2. The median operation time was 297 minutes, and the mean blood loss was 45 ml. Postoperative complications included Grade-2 aspiration pneumonia in 1 patient, and Grade-2 pancreatic fistula in another patient. The majority (18 of 26) of patients started oral feeding 4 days after the operation, and the median hospital stay was 13 days (Table 2). Twenty patients underwent a postoperative upper gastrointestinal series, which revealed no abnormalities. No patients complained of symptoms consistent with delayed gastric emptying during the follow-up period, and 12 patients underwent 1-year follow-up endoscopy, with a small amount of food debris found in only one patient.
While distal gastrectomy for malignancy is a common foregut procedure, principles involving the setting of the transection line are not well established. However, in subtotal gastrectomy for peptic ulcer disease, the standard transection line is well defined and extends from a point between the second last branch of the LGEA (greater curvature), to a point between the second last branch of the LGA (lesser curvature). As the purpose of subtotal gastrectomy for peptic ulcer disease is to resect the antrum and the body of the stomach to eradicate gastrin producing G and acid-secreting parietal cells, two-third to four-fifth of the stomach is resected, and this principle has been applied to radical distal gastrectomy for gastric cancer.
Resection of more than two-third of the stomach with adequate lymph node dissection is recommended by the Japanese gastric cancer treatment guidelines as a standard procedure in patients undergoing distal gastrectomy.3 Although Nomura et al.8 reported that operations in which only half of the stomach is removed can provide superior outcomes in terms of preservation of body weight in some early-stage cancer patients, from the viewpoint of oncological radicality, the Japanese gastric cancer treatment guidelines3 recommend resection of two-third of the stomach as a standard procedure. We followed this concept and performed routine resection of two-third of the stomach, by setting the transection line in patients in whom sufficient surgical margin is achieved.
However, setting the transection line is difficult, because of the variation in stomach shape, SGA and LGEA trajectory, and tumor location in each patient. These difficulties are compounded by the limited laparoscopic field of view, which complicates the identification of reliable anatomical landmarks. Moreover, inaccurate transection may lead to complications, as a too small remnant stomach would render gastroduodenostomy technically difficult because of the tension under which the anastomosis would have to be performed, and a too large remnant stomach may lead to postoperative gastric stasis. Since the greater curvature length of the remnant stomach has a greater impact on clinical outcomes than the lesser curvature length, the transection point along the greater curvature is extremely important, and reproducible technical results are therefore necessary.
Using our method, the proximal avascular area of the two landmarks can be identified in all patients without splenomegaly using the location of the lower pole of the spleen as a reference point. However, identification of the distal landmark, namely the communication point of the LGEA and RGEA, can be difficult in cases where these vessels are continuous. Takeda et al.9 reported in a study of human cadavers that communication between these vessels was absent and present in 38% and 62% of cases, respectively. In patients without vessel communication, the distal landmark can be easily identified and the origin of the transection line can be set at the center of these 2 landmarks along the greater curvature. However, in those with vessel communication, setting the origin of the transection line at the level of the second or third branch of the LGEA, away from the proximal landmark, provides similar results. Gentian violet ink was used for marking in our series, but laparoscopic clips may be also considered good alternatives, especially in obese patients to improve landmark visibility and to avoid ink fading during the operation.
Although the short-term outcomes in our study were satisfactory, a small amount of food debris was found in one patient on 1-year follow-up endoscopy. While the patient had no symptoms of delayed gastric emptying, we believe that further follow-up data collected via administration of a health-related quality of life questionnaire and/or inclusion of a larger number of patients might be necessary to establish firm conclusions on whether our procedure really provides long-term functional benefits.
With this technique, about two-third of the stomach was reproducibly resected under the limited laparoscopic field of view, and gastroduodenostomy with a delta-shaped anastomosis1,6 was safely performed without tension. Although further studies with a longerterm follow-up are necessary to confirm our findings, our method appeared not only theoretically secure based on the anatomy, but also led to reproducible results that were oncologically sound. We believe our simple and useful technique may serve as a guide for surgeons conducting LDG with appropriate resection line and ultimately could become a standard procedure.
Figures and Tables
Fig. 1
Overview of the stomach transection plan. The blue dots show the position of the proximal and distal anatomical landmarks to which Gentian violet ink is applied during laparoscopic distal gastrectomy. The proximal landmark is located in the avascular area between the last branch of the short gastric artery and the first branch of short gastric artery (LGEA), and the distal one, at the point of communication between the right gastroepiploic artery and the LGEA. The opened arrow shows the transection line. AGB = arteria gastricae breves; AGES = arteria gastroepiploica sinistra; APIS = arteria phrenica inferior sinistra; VGED =vena gastroepiploica dextra; VCDA =vena colica dextra accessoria; VCD = vena colica dextra; VCM = vena colica media. Data from the article of Japanese Gastric Cancer Association (Gastric Cancer 2011;14:101-112)7 with original copyright holder's permission.
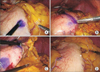
Fig. 2
Marking of the two stomach anatomical landmarks. (A) The avascular area between the last branch of the short gastric artery (SGA) and the first branch of the short gastric artery (LGEA) is marked with Gentian violet ink. The branches of the LGEA and SGA are indicated with white and dotted white arrows, respectively. The spleen dorsal to the omental fat is outlined by the black dotted line. (B) The white star shows the middle point between the right gastroepiploic artery (white arrow) and the LGEA (dotted white arrow). In this patient, there is no direct communication between the two arteries. (C) View of the stomach after dissection of the lymph nodes along the LGEA (No. 4sb). The dotted white line shows the SGA while the two landmarks appear clearly marked with Gentian violet ink. (D) Prior to stomach transection, the transection line is outlined with Gentian violet ink from the middle point between the two landmarks (dotted white circles) towards the lesser curvature.
References
1. Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002; 195:284–287.
2. Kanaya S, Haruta S, Kawamura Y, Yoshimura F, Inaba K, Hiramatsu Y, et al. Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc. 2011; 25:3928–3929.
3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
4. UICC International Union Against Cancer. TNM classification of malignant tumours. 7th ed. New York: Wiley;2009. p. 73–77.
5. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196.
6. Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, et al. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011; 14:365–371.
7. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.
8. Nomura E, Lee SW, Bouras G, Tokuhara T, Hayashi M, Hiramatsu M, et al. Functional outcomes according to the size of the gastric remnant and type of reconstruction following laparoscopic distal gastrectomy for gastric cancer. Gastric Cancer. 2011; 14:279–284.
9. Takeda FR, Cecconello I, Szachnowicz S, Tacconi MR, Gama-Rodrigues J. Anatomic study of gastric vascularization and its relationship to cervical gastroplasty. J Gastrointest Surg. 2005; 9:132–137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download