This article has been
cited by other articles in ScienceCentral.
Abstract
Juvenile polyps are the most common types of polyps in children, and patients usually present with lower gastrointestinal (GI) bleeding as the predominant symptom. These lesions, which are referred to as hamartomas, usually measure approximately 2 cm in size and are benign tumors located mainly in the rectum and sigmoid colon. The most common symptom of a juvenile polyp is mild intermittent rectal bleeding. It is rare for anemic patients because the amount of blood loss is small and often not diagnosed immediately. We present the case of a 6-year-old girl with a juvenile polyp in the distal transverse colon, who developed hypovolemic shock due to massive lower GI bleeding. Pediatricians must perform colonoscopy for thorough evaluation of polyps, because their location and size can vary and they can cause massive bleeding.
Keywords: Polyp, Children, Anemia, Colonoscopy
INTRODUCTION
Rectal and/or lower gastrointestinal (GI) bleeding in school-age or younger children is usually caused by infection, gastroenteritis, anal fistula, intussusception, and Meckel's diverticulum. Juvenile polyps are the second most common noninfectious cause of rectal bleeding after anal fistula [
1]. Polyps are suspected in a healthy-looking child who frequently passes small amounts of bright red stool, unaccompanied by pain. Although a single polyp is not common in children, most patients with a confirmed diagnosis usually show a juvenile hamartomatous polyp [
23]. The most common symptom of a juvenile polyp is mild intermittent rectal bleeding; however, Seo [
4] reported that 29% of patients also show anemia [
5]. The lesion is usually located in the rectum or sigmoid colon, although polyps can also occur in the descending, ascending, or transverse colon [
15]. We report an unusual case of a 6-year-old girl with a juvenile polyp located in the transverse colon, accompanied by hypovolemic shock due to massive bleeding. Additionally, we have reviewed the relevant literature.
CASE REPORT
A 6-year-old girl was admitted to the hospital in an unconsciousness state with a history of massive hematochezia earlier that morning. She experienced dizziness while walking and collapsed. Although she could stand thereafter, she collapsed immediately, and within a minute, she became pale and lost consciousness. During this episode, she simultaneously passed stool with a large volume of fresh blood that soaked her underwear and pants. The exact amount of bleeding was unknown; however, a small amount of additional bleeding was observed later. The patient was born at full term following a natural delivery, with a birth weight of 2.8 kg. Her weight at admission was 18 kg (10th percentile) and height was 117 cm (75th–90th percentile); her growth and development were normal. She had no family history of lower GI bleeding or polyps.
Bleeding was observed at the time of admission; this was only fresh blood without any feces, and the amount of blood lost soaked approximately one-third of a large adult-sized pad. She was alert, her blood pressure was 110/60 mmHg, pulse was 127 beats/min, respiratory rate was 24 breaths/min, and body temperature was 37.5°C. Her face and conjunctiva were markedly pale; however, no abnormal respiratory sounds were auscultated, and her heart sounds were regular. Her abdomen was soft and flat, although her bowel sounds were hyperactive. No hepatosplenomegaly, tenderness, or rebound tenderness was detected. She had not passed urine even once since waking that morning until the time of admission. Digital rectal examination revealed hematochezia; however, no hemorrhoids or other palpable masses were detected.
Blood tests showed the following results: white blood cells 7,300/μL, serum hemoglobin (Hb) 8.1 g/dL, hematocrit (Hct) 24.8%, mean corpuscular volume (MCV) 78.9 fL, mean corpuscular hemoglobin (MCH) 25.8 pg, reticulocytes 1.9%, platelets 387,000/μL, prothrombin time 13.3 s (international normalized ratio 1.2), activated partial thromboplastin time 26.5 s, serum C-reactive protein 2.7 mg/dL, blood urea nitrogen 14.2 mg/dL, serum creatinine 0.35 mg/dL, aspartate transaminase 21 IU/L, alanine transaminase 10 IU/L, and glucose 106 mg/dL.
Sigmoidoscopy was performed immediately after admission; however, the lesion could not be identified. On the 2nd day of hospitalization, we performed esophagogastroduodenoscopy and colonoscopy, and a polyp (3–4 cm) with a stalk was observed in the distal transverse colon, with no active bleeding. The polyp was round and erythematous and was covered with mucus (
Fig. 1). Abdominal computed tomography was performed for further evaluation of the lesion, and no lesions other than the pedunculated polyp were identified (
Fig. 2).
Fig. 1
Colonoscopy image showing the mass (A) and stalk (B). The mass was identified as a pedunculated solitary juvenile polyp with a smooth bright red and friable.
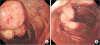
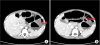
Fig. 2
(A) Heterogeneous polyp that has no calcification (arrow). (B) Stalk of polyp in the distal transverse colon (arrow).
On the 4th day of hospitalization, after transfusion of packed red blood cells, colonoscopy was performed using an electronic videoendoscope (PCF-240I; Olympus Optical, Tokyo, Japan), and monopolar snare cautery was used to excise the polyp, which was 4.5 cm long (
Fig. 3). The patient showed no further bleeding, and no complications were observed after colonoscopic polypectomy. Blood tests showed the following results: Hb 11.0 g/dL, Hct 33.5%, MCV 82.3 fL, MCH 27.0 pg, and platelets 400,000/μL.
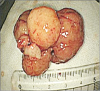
Fig. 3
A 4.5 cm polyp after polypectomy.
Histopathological examination performed on the 8th day of hospitalization confirmed that the lesion was a juvenile polyp (
Fig. 4). The patient was discharged following improvement in her symptoms and stabilization of vital signs. Follow-up colonoscopy was not performed because no further symptoms were observed after discharge.
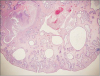
Fig. 4
Microscopic photograph revealing a cystically dilated gland, excess lamina propria, and dense infiltration of inflammatory cells (H&E, ×75).
DISCUSSION
Juvenile polyps are usually diagnosed in children aged 6 years and are more prevalent in boys [
6]. The 6-year-old girl described in this report showed a typical presentation of juvenile polyps with hematochezia unaccompanied by abdominal pain. However, in contrast to the presentation of most juvenile polyps, she developed syncope and severe anemia (Hb 8.1 g/dL) due to acute massive bleeding. Kim et al. [
6] reported that anemia is rare, and 12.2% of children diagnosed with juvenile polyps showed mild anemia, with serum Hb of 11.0 g/dL. However, a previous report has described massive bleeding in a patient who presented with severe anemia (Hb 8.9 g/dL), similar to the present case [
6]. Cynamon et al. [
7] reported that patients with juvenile polyps showed a 33% higher rate of hypochromic microcytic anemia; however, no patient in their cohort required a blood transfusion. Juvenile polyps with iron deficiency anemia (IDA) show a low probability of malignant transformation, although surgical removal is required in symptomatic cases [
8]. In a study performed by Cheon et al. [
9], 4 of 19 patients with juvenile polyps developed anemia, with severe anemia (serum Hb 5.9–6.8 g/dL) observed in a few cases. However, all patients recovered without blood transfusion after surgical removal of the polyp followed by supplemental iron administration [
9].
Previous reports have described that IDA in patients with juvenile polyps could primarily be attributed to recurrent mild bleeding. However, the juvenile polyp caused significant acute lower GI bleeding in our patient even in the absence of a past history of IDA or a history of recurrent mild bleeding. Except for anemia caused by lower GI bleeding, our limitation is that no further examination was performed to investigate other causes of syncope in this patient.
Usually, the mean size of polyps is 1–3 cm with diverse morphology; however, Kim et al. [
10] reported that polyps are commonly 0.6–1 cm in size. The largest polyp detected in their study was 3.5 cm and they were often round and erythematous [
11]. Other studies have reported round and erythematous polyps measuring 0.3–2 cm in size [
12]. Matsushita et al. [
13] described the removal of a juvenile polyp (2.7 cm) with a thick stalk, which was identified in the descending colon in a 1-year-old infant. Although the polyp discussed in this report was larger, it could be removed endoscopically because of its pedunculated morphology and shape that resembled other polyps.
In most cases, single juvenile polyps are located in the rectum or sigmoid colon; however, some may occur near the proximal large intestine [
791415]. In our case, we performed initial sigmoidoscopy, followed by colonoscopy to identify the lesion. Although the rectum and the sigmoid colon are the most common locations, polyps are known to occur at various sites in cases of multiple and even in some cases of single lesions. Therefore, researchers stated the necessity of total colonoscopy even in cases demonstrating rectal bleeding [
14].
Histopathologically, the polyp was a benign hamartoma with a very low probability of malignant or adenomatous transformation. However, a previous study reported that among 36 patients diagnosed with juvenile polyps, adenomatous transformation occurred in only one patient (2.7%), although only low-grade dysplasia was observed [
6].
We endoscopically excised the polyp using intravenous ketamine for sedation. Notably, general anesthesia is required in some cases, particularly in younger patients. For example, Jalihal et al. [
14] reported that most polyps in children aged <3 years could be removed without general anesthesia.
Juvenile polyps show a relatively low relapse rate; however, Pillai and Tolia [
15] reported a relapse rate of 7.9% in the first 8 months–10 years after initial diagnosis. Notably, Mestre [
16] and Cynamon et al. [
7] reported relapse rates of 9% and 17% respectively. In our case, the patient has shown no signs of relapse even a year after polypectomy.
In conclusion, juvenile polyps do not usually cause acute and severe bleeding; however, significant or prolonged bleeding can lead to anemia. As mentioned earlier, clinicians should consider juvenile polyps as well as other causes in patients with severe lower GI bleeding and consequent anemia. We report an unusual case of a patient with a juvenile polyp located in the transverse colon, accompanied by massive bleeding resulting in hypovolemic shock.






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download