Abstract
Collagenous gastritis (CG) is a rare disorder that is characterized by the presence of a thick subepithelial collagen band with multiple infiltrated inflammatory cells of the gastric mucosa. CG is divided into two major subsets: first, in children and young adults presenting with severe anemia and abdominal colic pain (pediatric-type CG); and second, in adult patients with chronic watery diarrhea associated with collagenous colitis (adult-type CG). We report two cases of pediatric-type CG, each presenting with refractory anemia and chronic diarrhea.
Collagenous gastritis (CG) is a rare disorder of unknown origin. CG is diagnosed by endoscopic gastric mucosal biopsy and is characterized by a subepithelial collagenous band deposit greater than 10 µm with various degrees of inflammatory cell infiltration [1,2]. The thickened collagenous bands can entrap dilated capillaries and these bands are usually identified as mixed chronic inflammation in the lamina propria and surface epithelial damage of varying severity on gastric biopsy. In children and young adults, presenting symptoms usually include severe colicky abdominal pain and severe iron deficiency anemia without collagenous colitis (CC). In adults the combination of CG and CC is relatively common with non-hemorrhagic watery diarrhea being the most common symptom. Thus, Lagorce-Pages et al. [3] have delineated two subsets in patients with CG: pediatric-type CG and adult-type CG. Most cases of CG have presented with pediatric-type CG; however, Leiby et al. [4] first reported an adult-type CG in a 2-year-old child with chronic non-hemorrhagic watery diarrhea.
Irregular hyperemia, friability, and nodularity are the common endoscopic findings of CG, while there is no typical finding of CC. The etiology, pathophysiology, treatment and the prognosis of CG are not well known yet.
Since Colletti and Trainer [1] first described a 15-year-old girl with CG in 1989. Park et al. [5] first reported an 11-year-old Korean pediatric CG patient with severe anemia. Here we report two cases of pediatric CG. The first case presented with typical pediatric-type CG feature and the second case presented with chronic diarrhea similar to adult-type CG, yet CC was insufficient as a diagnosis.
A 7-year-old girl visited the clinic due to fatigue, shortness of breath, and a pale appearance. She had been diagnosed with iron deficiency anemia 5 months earlier and had taken oral iron supplements for 1 month without follow up. She did not complain of any other gastrointestinal symptoms. Her height and weight were 127 cm (75-90 percentile) and 24 kg (50-75 percentile), respectively, and her physical exam was clear except for pale conjunctiva. A blood laboratory test showed a hemoglobin (Hb) 6.2 g/dL, mean corpuscular volume (MCV) 55.7 fL, white blood cell (WBC) 4,400/mm3 with normal differentiation, iron 15 µg/dL, total iron binding capacity (TIBC) 429 µg/dL and ferritin 0.5 ng/mL. Multiple stool occult blood tests were negative. Her anemia did not respond to 4 weeks of oral iron supplementation, she underwent endoscopy and multiple diffuse flat elevated mucosal changes were identified in the entire gastric mucosa especially on the antrum. The test of Helicobacter pylori was negative. The endoscopic finding of esophagus, duodenum, small intestine and colon was all clear. The gastric mucosal biopsy revealed various degrees of plasma cell, eosinophil and neutrophil infiltration in the lamina propria with amorphous collagen bands of irregular thickness deposited in the subepithelial lamina propria of the gastric antrum and the surface epithelium was focally detached. The colonic mucosal biopsy was nonspecific. Two weeks of treatment with a proton pump inhibitor did not seem to influence her anemia. She underwent a transfusion after which her Hb rose up to 9.3 g/dL and did not further rise after use of oral iron. Her follow-up endoscopy, 3 months after the initial one, revealed further aggravated nodularity with a small amount of covered exudative material on the mucosa (Fig. 1). The biopsy finding still remained as various types of inflammatory cell infiltration with subepithelial collagen bands (Fig. 2).
Because of her refractory anemia, we considered that it might be due to impairment in iron absorption. After injecting 7 mg/kg of intravenous iron sucrose, her Hb rose to 10 g/dL, and thus she undertook injections of intravenous iron sucrose intermittently. Currently she continues to maintain her Hb level at 12 g/dL.
An 8-year old boy presented to our clinic due to chronic diarrhea. He had a history of chronic diarrhea, paralytic ileus, and failure to thrive at the age of 2. The endoscopic finding at that time was not specific except for diffuse nodularity in the duodenal mucosa and pathology testing revealed Helicobacter pylori-related chronic gastritis and chronic nonspecific colitis. His clinical presentation pointed to a diagnosis of autoimmune enterocolitis. He underwent steroid therapy for several weeks and the paralytic ileus improved. After that time, he suffered from chronic diarrhea two times per year and his iron deficiency anemia persisted at a mild degree for 6 years. The symptoms partially improved after intravenous hydration and oral iron supplement.
He was admitted for 2 weeks for watery diarrhea and vomiting with dehydration. He looked sick and chronically ill. He exhibited stunted height and weight (128 cm [25-50 percentile] and 19.3 kg [<3 percentile], respectively). His weight had decreased 1.7 kg during the course of 2 weeks. His laboratory tests revealed Hb 10.4 g/dL, MCV 5.5 fL, WBC 9,600/mm3 with normal differentiation, iron 21 µg/dL, TIBC 349 µg/dL, ferritin 1.0 ng/mL, which suggested iron deficiency anemia. Other blood and stool tests did not result in any significance findings.
He underwent endoscopy, which identified diffuse hyperemia, multiple, broad-based, elevated mucosal changes with covered exudative materials on the entire gastric mucosa. The mucosa was consistent with multiple chronic inflammatory cell infiltrations with scattered thick subepithelial collagenous bands, which was sufficient to diagnose with CG. And his colonic mucosa also identified multiple lymphocyte and neutrophil infiltrations with thin subepithelial collagenous bands from the terminal ileum to the sigmoid colon (Fig. 3). However the thickness of collagenous bands on colonic mucosa was insufficient to satisfy the testing necessary for a diagnose with CC. His colonoscopy was grossly normal. There was neither evidence of Helicobacter pylori infection in the stomach nor infectious colitis. He was thought to have adult-type CG like feature, even though a diagnosis of CC had not yet been satisfied. His anemia and diarrhea improved after oral iron supplementation and supportive care.
There have been 14 cases reported of CC in patients less than 18 years old until 2011. Including our two cases, the gender was slightly female dominant (males 4, females 10, unknown 2) [1,3,5-8]. On the other hand, three reported cases of adult-type CC were all male patients, including our second case. The onset age was 2 to 16 years old, and the cases did not have combined infectious gastroenteritis, celiac sprue, or inflammatory bowel disease. The use of mefenamic acid was reported for one patient who was suffering from dysmenorrhea. However, her CG was continuously aggravated after discontinuing the medication [6]. All except one case among the 13 pediatric-type CG cases had combined iron deficiency anemia, seven patients suffered from severe colicky abdominal pain, and diarrhea was involved in just one case. Three cases of adult-type CG were accompanied by watery diarrhea, two showed iron deficiency anemia, and none presented abdominal pain. CC has been reported to have a possible connection with celiac sprue, inflammatory bowel disease, and/or systemic disorders such as thyroid disease in adults [6,9,10], whereas CG has not been associated with other disorders. For this reason, there have been controversies whether CG is a single disorder or a presentation of a systemic disorder.
The main pathogenesis involved in the development of iron deficiency anemia in CG was thought to be chronic loss of blood via abnormally dilated capillaries entrapped within collagenous bands [2]. There has been no reported gross loss of blood, most of cases were of combined iron deficiency anemia, and they improved after oral iron supplement. Therefore, the cause of anemia is supported by loss of blood. However, there has been another case which did not respond to oral iron supplement but did improve after intravenous iron supplementation, like our first case [8]. Generally, the oral iron absorption rate has been thought to be 10% of the oral iron supplement and in severe iron deficiency case, up to 20% of iron can be absorbed. In our first case, we continued oral iron supplement for several months without response, but only one dose of intravenous iron supplementation was able to increase the reticulocyte and Hb levels. There has been an increasing awareness of non-bleeding gastrointestinal disorders that may result in the impairment of iron absorption like in celiac disease, Helicobacter pylori infection, autoimmune atrophic gastritis and hereditary iron-refractory iron deficiency anemia [11,12]. Like our first case, decreased iron absorption could be one of the important causes of iron deficiency anemia in such gastrointestinal conditions. It has been circumspect with the use of a proton pump inhibitor which can decrease gastric acidity, and the elimination of the suspected inhibitor of iron absorption could also be attempted in such patients.
Winslow et al. [13] reported the clinicopathologic evaluation of CG in 109 biopsy specimens from 19 different endoscopic procedures in a patient over a 12-year period. It showed a significantly lower number of antral gastrin cells, along with a significant corpus endocrine cell hyperplasia during the follow-up period, which were compared to age- and sex-matched control subjects. The gastric corpus biopsy specimens also revealed an active, chronic gastritis, subepithelial collagen deposition, smooth muscle hyperplasia, and mild-to-moderate glandular atrophy. These results suggested the possibility of a developing endocrine tumor or adenocarcinoma. The unpublished data of Park et al. [5] revealed the similar clinicopathologic feature of the first examination in every year's endoscopic follow up during a 6-year period. To date, there have been few reports of improving CG, including adult reports. The disease might have the potential to become chronic and an aggravated disorder with increasing age.
CG is a very rare disorder, with a not well-established etiology, pathogenesis, treatment, or prognosis. It has not yet been concluded whether CG is a single disorder or one of a series of presenting symptoms in systemic disorder. Also it has not been shown whether pediatric-type CG and adult-type CG come from same etiology or not. It will be more fruitful to have further trial groupings and longer period follow-ups towards the development of a further understanding of CG.
Figures and Tables
Fig. 1
Upper gastrointestinal endoscopic examination showing diffuse mucosal hyperemia and multiple diffuse flat elevated mucosal changes of the antrum (A) and body (B) of the stomach (case 1).

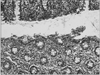
Fig. 2
Collagenous gastritis (case 1). (A) Hematoxylline-eosin stain, original magnification (×200). (B) Masson-trichrome stain, original magnification (×200). Amorphous collagen bands of irregular thickness deposited in the subepithelial lamina propria of the gastric antrum (arrows). Surface epithelium is focally detached (arrowhead), and accompanied by lymphoplasmacytic infiltration in the lamina propria.

References
2. Côté JF, Hankard GF, Faure C, Mougenot JF, Holvoet L, Cézard JP, et al. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol. 1998. 29:883–886.

3. Lagorce-Pages C, Fabiani B, Bouvier R, Scoazec JY, Durand L, Flejou JF. Collagenous gastritis: a report of six cases. Am J Surg Pathol. 2001. 25:1174–1179.
4. Leiby A, Khan S, Corao D. Clinical challenges and images in GI. Collagenous gastroduodenocolitis. Gastroenterology. 2008. 135:17. 327.
5. Park S, Kim DH, Choe YH, Suh YL. Collagenous gastritis in a Korean child: a case report. J Korean Med Sci. 2005. 20:146–149.

6. Brain O, Rajaguru C, Warren B, Booth J, Travis S. Collagenous gastritis: reports and systematic review. Eur J Gastroenterol Hepatol. 2009. 21:1419–1424.

7. Suskind D, Wahbeh G, Murray K, Christie D, Kapur RP. Collagenous gastritis, a new spectrum of disease in pediatric patients: two case reports. Cases J. 2009. 2:7511.

8. Kori M, Cohen S, Levine A, Givony S, Sokolovskaia-Ziv N, Melzer E, et al. Collagenous gastritis: a rare cause of abdominal pain and iron-deficiency anemia. J Pediatr Gastroenterol Nutr. 2007. 45:603–606.

9. Freeman HJ. Collagenous mucosal inflammatory diseases of the gastrointestinal tract. Gastroenterology. 2005. 129:338–350.

10. Macaigne G, Boivin JF, Harnois F, Chayette C, Dikov D, Cheaib S, et al. Collagenous gastritis and ileo-colitis occurred in autoimmune context: report of a case and review of the literature. Gastroenterol Clin Biol. 2010. 34:e1–e6.
11. Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, et al. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. 2003. 52:496–501.

12. Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, Helicobacter pylori, and autoimmune gastritis. Semin Hematol. 2009. 46:339–350.

13. Winslow JL, Trainer TD, Colletti RB. Collagenous gastritis: a long-term follow-up with the development of endocrine cell hyperplasia, intestinal metaplasia, and epithelial changes indeterminate for dysplasia. Am J Clin Pathol. 2001. 116:753–758.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download