Abstract
Background/Aims
We recently identified recessive mutations in the solute carrier organic anion transporter family member 2A1 gene (SLCO2A1) as causative variants of chronic enteropathy associated with SLCO2A1 (CEAS). The aim of this study was to evaluate SLCO2A1 protein expression in the intestinal tissues of patients with CEAS, intestinal Behçet's disease (BD), simple ulcer (SU), and Crohn's disease (CD).
Methods
Immunohistochemical staining using a polyclonal anti-SLCO2A1 antibody was performed on the resected intestinal specimens from 13 cases of CD, 9 cases of intestinal BD/SU, and 3 cases of CEAS. The extent of SLCO2A1 expression was determined by counting positively-staining vascular endothelial cells and scored as 0 (no cells), 1 (1%–30% cells), 2 (31%–60%), or 3 (>60%). The intensity of SLCO2A1 expression was scored either as 0 (negative), 1 (intermediate), or 2 (strong). The extent score and intensity score were summed for the final score of 0, 2, 3, 4, or 5.
Results
SLCO2A1 protein expression was observed in 1 of 3 cases of CEAS (33%), all 13 cases of CD (100%), and all 9 cases of BD/SU (100%). The mean final expression scores of CEAS, CD, and BD/SU were 1.6 (range, 0–5), 4.8 (range, 4–5), and 4.3 (range, 4–5), respectively. The final expression score in CEAS was significantly lower than in CD (P=0.03).
Chronic nonspecific multiple ulcers of the small intestine (CNSU) is a rare autosomal recessive inherited disease characterized by chronic loss of blood and protein through persistent, intractable, nonspecific small intestinal ulcers.1
2
3
4 We recently identified the causative gene for CNSU, the solute carrier organic anion transporter family member 2A1 gene (SLCO2A1), which encodes a PG E2 transporter protein.5
SLCO2A1 has also previously been reported as a causative gene for a subtype of primary hypertrophic osteoarthropathy (PHO).6
7
8 We clarified that SLCO2A1 protein is normally expressed on vascular endothelial cell membranes in small intestinal tissues, but these tissues have completely deteriorated in most patients with CNSU.5 Based on these findings, we proposed a more appropriate nomenclature of chronic enteropathy associated with SLCO2A1 (CEAS) for this disease.5 However, the gastrointestinal expression of SLCO2A1 protein in patients with various IBDs other than CEAS has not yet been reported.
The clinical and histological distinction of CEAS from other IBDs, especially CD, is a matter of some debate. The small bowel is affected by chronic and intractable ulcers in both CEAS and CD. In fact, a family pedigree of enteropathy was reported as CD, although the actual diagnoses presumably should have been CEAS.9
In the present study, we evaluated the immunohistochemical expression of SLCO2A1 protein in intestinal tissues from patients with IBDs, including CEAS, CD, and Behçet's disease (BD) or simple ulcer (SU).
We reviewed the clinical files of patients diagnosed with IBDs at our institutions between 2001 and 2014. Out of this group, 25 patients (13 CD, 9 BD/SU and 3 CEAS), had formalin-fixed and paraffin-embedded surgically resected intestinal tissues (ileum and/or colon) available and were enrolled in the current study. The diagnoses of CD and intestinal BD were based on the established Japanese criteria.10
11 The diagnoses of SU were based on the presence of deep discrete ulcerations with punched-out, round or oval sharp margins in the ileocecal region that showed macroscopic and histological similarities to intestinal BD, but clinically not fulfilling the criteria for BD.12
13 The study protocol was approved by the Ethics Committee at Iwate Medical University Hospital (H28-59), and the study was conducted in accordance with the Helsinki Declaration. Written informed consents were obtained from all patients.
Immunohistochemical staining was performed on 4-µm-thick sections cut from each of the formalin-fixed and paraffin-embedded tissue blocks with SLCO2A1 polyclonal antibody (HPA013742; Sigma-Aldrich, St. Louis, MO, USA) and CD31 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) using the Dako Envision system (Dako, Glostrup, Denmark). Immunoreactivity for SLCO2A1 on the vascular endothelial cells, as confirmed by CD31 immunostaining, was evaluated in the mucosa and submucosa of the intestinal tissues, including both ulcerative lesions and normal-appearing tissues without ulcerations. The extent and intensity of staining was judged independently by 2 of the authors (S.Y. and T.S.).
The intensity of SLCO2A1 expression was scored either as 0 (negative), 1 (intermediately positive), or 2 (strongly positive), as shown in Fig. 1. Thus, intensity scores 1 and 2 were defined as positive. The extent of SLCO2A1 expression in vascular endothelial cells in the respective lesions was scored either as 0 (no cells staining), 1 (1%–30% of cells staining positive), 2 (31%–60%), or 3 (>60%), based on vascular endothelial cell expression of SLCO2A1 in the respective lesions. The final expression score was defined as the extent score plus the intensity score, and was scored as 0, 2, 3, 4, or 5.14
15 In each patient, at least 3 sections, including ulcerative lesions and normal-appearing tissues, were investigated by immunohistochemistry.
The clinicopathologic characteristics of all patients are summarized in Table 1. The median age at diagnosis of patients with CD (23 years) and CEAS (22 years) seemed younger than those with BD/SU (51 years), but there was no statistically significant difference among the 3 groups. While patients with CD were predominantly male, those with BD/SU and CEAS were predominantly female. The ileocecal region was examined in 69% of CD cases and all BD/SU cases, while the ileum was examined in all CEAS cases.
Table 2 shows details of SLCO2A1 mutations in the 3 patients with CEAS. In 2 patients (cases 1 and 2), a homozygous mutation was detected in exon 7 and exon 10, respectively. The remaining patient (case 3) had compound heterozygous mutations in exons 5 and 13.
There was positive immunostaining of SLCO2A1 in all patients with CD (Fig. 2A) and BD/SU (Fig. 2B and C), but only in 1 of the 3 patients with CEAS (33%, Table 1). Specimens from CEAS cases 1 and 2 were negative for SLCO2A1 expression in vascular endothelial cells in the mucosa and submucosa of both the ulcerative lesions and normal-appearing tissues (Fig. 2D and E); However, those from CEAS case 3 stained positive for SLCO2A1 expression in vascular endothelial cells in the mucosa and submucosa of both the ulcerative lesions and normal-appearing tissues (Fig. 2F). Both intensity and extent scores did not differ between ulcerative lesions and normal-appearing tissues in all 3 cases of CEAS.
The SLCO2A1 expression scores in the 3 groups are shown in Fig. 3. The mean final score in patients with CEAS (1.7) was lower than in patients with CD (4.8) or BD/SU (4.3) (Fig. 3C). The final score in CEAS was significantly lower than in CD (P<0.05), but not in BD/SU (P=0.23). In patients with BD/SU, the intensity of SLCO2A1 immunoreactivity in ulcerative lesions was weaker than that in normal lesions (Fig. 2B and C). The mean final score in areas with ulcerative lesions was significantly lower than that in areas with normal-appearing tissue (4.2 vs. 4.8, P<0.05) (Fig. 4). In patients with CD and CEAS, however, no difference was observed: staining patterns, including extent and intensity scores, did not differ between ulcerative lesions and normal-appearing tissues.
The macroscopic characteristics typical of intestinal ulcerative lesions in various IBDs are well documented. Longitudinal ulcers and cobblestone appearance are characteristic of CD, while round or oval-shaped, deep punched-out ulcers with clearly demarcated margins are characteristic of intestinal BD/SU. In contrast, multiple sharply demarcated shallow ulcers with an irregular circular or linear shape are characteristic of CEAS (CNSU). However, it is sometimes difficult to correctly diagnose these diseases with endoscopy or radiography alone.
In 1997, Compton et al.9 reported 3 cases of familial CD combined with PHO. Similar cases of PHO accompanied by CD had been reported previously.16
17 We suggest that these cases should have been diagnosed as CEAS rather than CD, since CEAS and PHO share a causative gene.5 Furthermore, we previously reported that 2 of 603 patients with a clinical diagnosis of CD were actually found to have compound heterozygous mutations in SLCO2A1,5 and on this basis, the diagnosis for these 2 patients was revised to CEAS. There are clearly several instances of CEAS cases being misdiagnosed as CD probably because they shared common clinical features like small intestinal ulcers, anemia, and hypoalbuminemia.5 Genetic analysis is therefore necessary to distinguish CEAS from CD.
In the present study, we have shown that immunohistochemical staining for SLCO2A1 protein is useful to distinguish CEAS from CD and BD/SU. While the immunoexpression of SLCO2A1 protein was positive in all cases of CD and BD/SU, it was negative in 2 of 3 cases of CEAS. The CEAS case with positive SLCO2A1 expression (case 3) had compound heterozygous mutation in exons 5 and 13 of the SLCO2A1 gene (Table 2). Although the complete SLCO2A1 protein is 643 amino acids long, the antibody used in the current study (HPA013742) recognizes only the 83 amino acids of the fifth extracellular domain coded by exons 9-11 (431-513) (The Human Protein Atlas, http://www.proteinatlas.org/ENSG00000174640-SLCO2A1/antibody).5 Because of the compound heterozygous mutations in case 3, the full-length SLCO2A1 protein might have been synthesized and recognized by the antibody (Fig. 5). CEAS case 1 and 2 had homozygous mutations and the length of the synthesized SLCO2A1 proteins would be too short for detection by this antibody. These truncated mutant alleles have been reported to account for approximately 60% of all mutated alleles found in patients with CEAS;5 so, more than 60% of cases of CEAS can be correctly diagnosed only by observing a loss of expression via immunohistochemistry (Table 2).
It is also interesting that the final SLCO2A1 expression score in areas around ulcerative lesions was significantly lower than in areas of normal-appearing tissues in BD/SU (Figs 2B, C and 4). We speculate that the severe inflammation around the ulcerative lesions might cause a decrease in the expression of SLCO2A1 protein.
The present study has several limitations. First, genetic analysis for SLCO2A1 mutations was not performed in the CD or BD/SU cases; so, there is a possibility that CEAS cases might have been included in these groups. Second, we carried out the present study with a limited sample size in only a single center. Additional multicenter studies with a larger number of patients with clinically and genetically specified diagnoses of IBDs are warranted.
In conclusion, we suggest that immunohistochemical staining of SLCO2A1 expression is a useful tool in differentiating between a diagnosis of CEAS or other IBDs.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Tadakazu Hisamatsu, Kyorin University, Mitaka, Japan for his valuable comments on the immunohistochemical study of SLCO2A1 protein.
Notes
FINANCIAL SUPPORT: This work was supported in part by a grant from the Japan Agency for Medical Research and Development (AMED) (15ek0109053h0002).
AUTHOR CONTRIBUTION: Conceptualization: JU, MoE, TM, Methodology: ShY, MaE, NU, TS. Formal analysis: SaY, ShY. SN. Funding acquisition: TM. Project administration: SaY, ShY, SN, KK. Visualization: ShY, SN. Writing - original draft: SaY, ShY. Writing - review and editing: SN, TM. Approval of final manuscript: all authors.
References
1. Matsumoto T, Iida M, Matsui T, et al. Non-specific multiple ulcers of the small intestine unrelated to non-steroidal anti-inflammatory drugs. J Clin Pathol. 2004; 57:1145–1150. PMID: 15509673.

2. Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007; 66(3 Suppl):S99–S107. PMID: 17709045.

3. Matsumoto T, Kubokura N, Matsui T, Iida M, Yao T. Chronic nonspecific multiple ulcer of the small intestine segregates in offspring from consanguinity. J Crohns Colitis. 2011; 5:559–565. PMID: 22115375.

4. Esaki M, Umeno J, Kitazono T, Matsumoto T. Clinicopathologic features of chronic nonspecific multiple ulcers of the small intestine. Clin J Gastroenterol. 2015; 8:57–62. PMID: 25788296.

5. Umeno J, Hisamatsu T, Esaki M, et al. A hereditary enteropathy caused by mutations in the SLCO2A1 gene, encoding a prostaglandin transporter. PLoS Genet. 2015; 11:e1005581. DOI: 10.1371/journal.pgen.1005581. PMID: 26539716.

6. Zhang Z, Xia W, He J, et al. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet. 2012; 90:125–132. PMID: 22197487.

7. Busch J, Frank V, Bachmann N, et al. Mutations in the prostaglandin transporter SLCO2A1 cause primary hypertrophic osteoarthropathy with digital clubbing. J Invest Dermatol. 2012; 132:2473–2476. PMID: 22696055.

8. Diggle CP, Parry DA, Logan CV, et al. Prostaglandin transporter mutations cause pachydermoperiostosis with myelofibrosis. Hum Mutat. 2012; 33:1175–1181. PMID: 22553128.

9. Compton RF, Sandborn WJ, Yang H, et al. A new syndrome of Crohn's disease and pachydermoperiostosis in a family. Gastroenterology. 1997; 112:241–249. PMID: 8978365.

10. Hisabe T, Hirai F, Matsui T, Watanabe M. Evaluation of diagnostic criteria for Crohn's disease in Japan. J Gastroenterol. 2014; 49:93–99. PMID: 23546557.

11. Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet's disease: indication of anti-TNFalpha monoclonal antibodies. J Gastroenterol. 2014; 49:156–162. PMID: 23955155.

12. Muto T. Historical review of so-called “simple ulcer” of the intestine. Stomach Intestine. 1979; 14:739–749.
13. Matsukawa M, Yamasaki T, Kouda T, Kurihara M. Endoscopic therapy with absolute ethanol for postoperative recurrent ulcers in intestinal Behçe's disease, and simple ulcers. J Gastroenterol. 2001; 36:255–258. PMID: 11324729.

14. Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993; 85:200–206. PMID: 8423624.

15. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17:1474–1481. PMID: 10334533.

16. Shim YW, Suh JS. Primary hypertrophic osteoarthropathy accompanied by Crohn's disease: a case report. Yonsei Med J. 1997; 38:319–322. PMID: 9409195.

17. McAllister C, McNulty JG, Fielding JF. Is hypertrophic osteoarthropathy really so rare in regional enteritis? J Clin Gastroenterol. 1986; 8:562–566. PMID: 3782755.

Fig. 1
Examples of SLCO2A1 expression intensity scores of vascular endothelial cells. (A) Strong (score 2). (B) Intermediate (score 1). (C) Negative (score 0). Dako Envision detection kit, original magnification, ×200.
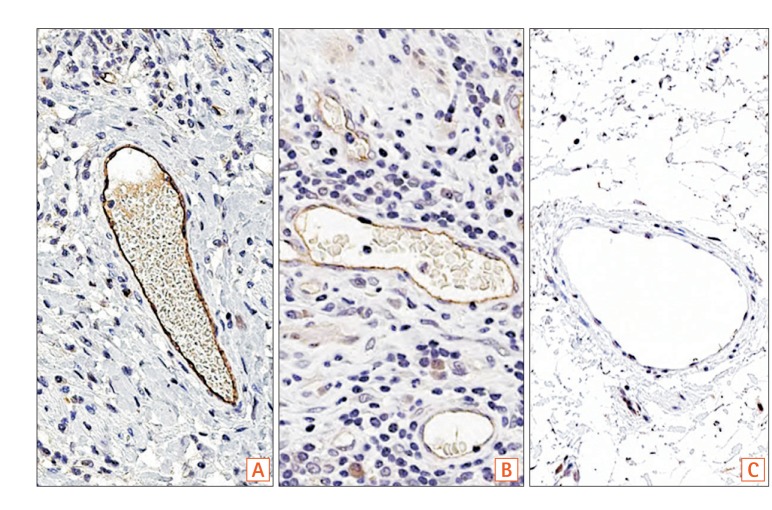
Fig. 2
(A) Immunostaining of SLCO2A1 in a case of CD (57 years old at surgery, male). Submucosal areas in normal-appearing ileal tissue adjacent to the ulcerative lesion. Extent score=3, intensity score=2, final score=5 (original magnification, ×100). (B, C) Immunostaining of SLCO2A1 in a case of BD (60 years old at surgery, female, original magnification, ×400). (B) Tissues from ulcerative lesion in the submucosa. Extent score=3, intensity score=1, final score=4. (C) Submucosal areas of normal-appearing colonic tissue adjacent to the ulcerative lesions. Extent score=3, intensity score=2, final score=5. (D-F) Immunostaining of SLCO2A1 in 3 cases of CEAS (original magnification, ×200). (D) Case 1: 34 years old at diagnosis, 50 years old at surgery, female. Extent score=0, intensity score=0, final score=0. (E) Case 2: 22 years old at diagnosis, 40 years old at surgery, female. Extent score=0, intensity score=0, final score=0. (F) Case 3: 22 years old at diagnosis, 27 years old at surgery, female. Extent score=3, intensity score=2, final score=5. CEAS, chronic enteropathy associated with SLCO2A1.
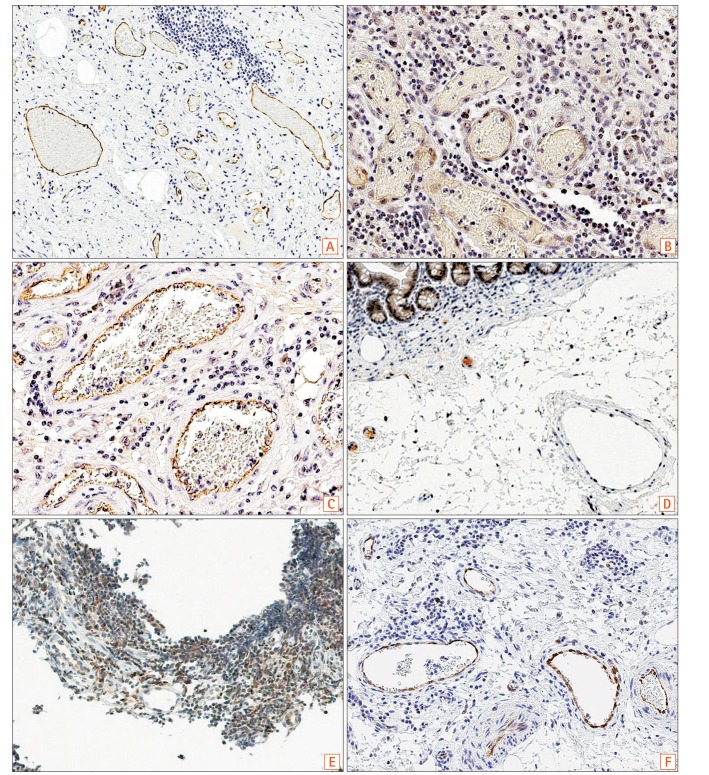
Fig. 3
The scores of SLCO2A1 expression in each group. (A) Extent scores: significant difference was observed between cases of CD and CEAS (P<0.05). (B) Intensity scores: significant difference was observed between cases of CD and CEAS (P<0.005), and between cases of BD/S and CEAS (P<0.05). (C) Final scores: significant difference was observed between cases of CD and CEAS (P<0.05), but not between cases of BD/SU and CEAS (P=0.23). BD, Behçet's disease; SU, simple ulcer; CEAS, chronic enteropathy associated with SLCO2A1.
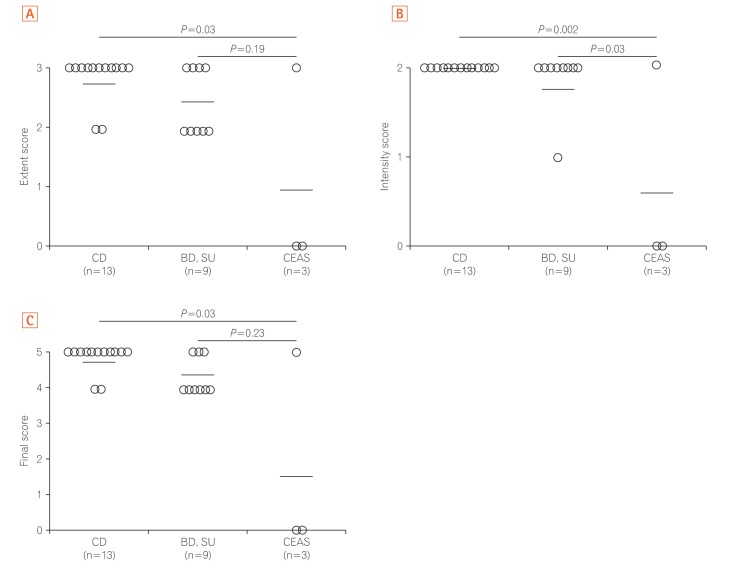
Fig. 4
Final scores of SLCO2A1 staining in normal-appearing tissues and ulcerative lesions in cases of BD/SU. A significant difference was observed between the 2 groups (P<0.05). BD, Behçet's disease; SU, simple ulcer.
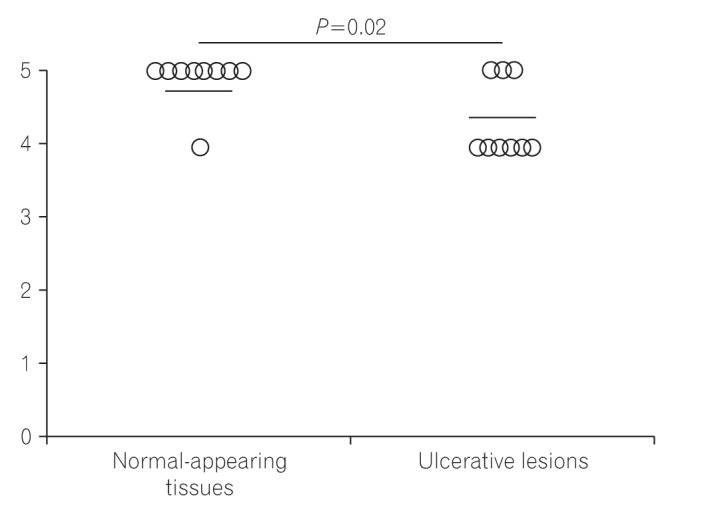
Fig. 5
Structures of wild-type SLCO2A1 proteins and CEAS mutations. While the full length of the SLCO2A1 protein is 643 amino acids (AAs), the antibody HPA013742 recognizes the 83 AAs of the 5th extracellular domain coded by exons 9-11 (431-513, http://www.proteinatlas.org/ENSG00000174640-SLCO2A1/antibody). In cases 1 and 2, the length of the synthesized SLCO2A1 protein was considered too short for detection by this antibody. Case 3 had the compound heterozygous mutation and the full length SLCO2A1 protein was synthesized and detected by HPA013742.
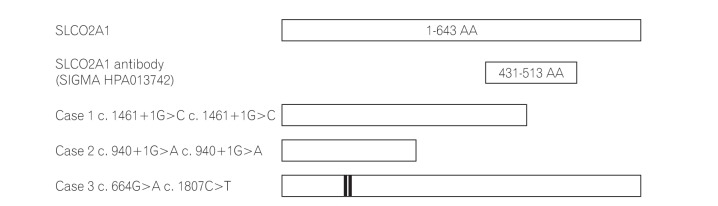
Table 1
Clinicopathologic Features of Study Patients
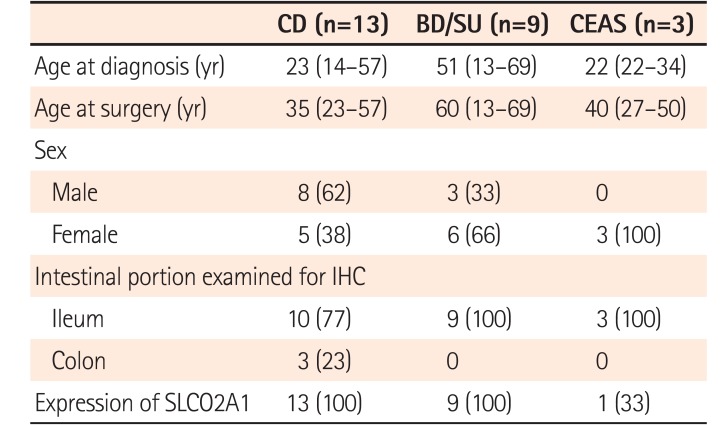
Table 2
SLCO2A1 Mutations in 3 Patients with CEAS
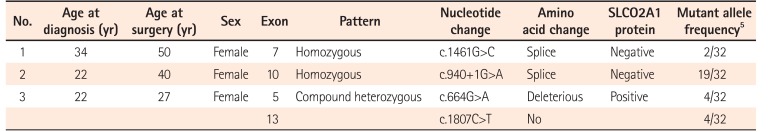
| No. | Age at diagnosis (yr) | Age at surgery (yr) | Sex | Exon | Pattern | Nucleotide change | Amino acid change | SLCO2A1 protein | Mutant allele frequency5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | 50 | Female | 7 | Homozygous | c.1461G>C | Splice | Negative | 2/32 |
| 2 | 22 | 40 | Female | 10 | Homozygous | c.940+1G>A | Splice | Negative | 19/32 |
| 3 | 22 | 27 | Female | 5 | Compound heterozygous | c.664G>A | Deleterious | Positive | 4/32 |
| 13 | c.1807C>T | No | 4/32 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download