Various pathogenic factors responsible for IBD have been studied; however, the immunopathogenesis of IBD is still unclear. In this issue of Intestinal Research, I read an interesting manuscript by Lee et al.1 “Immunological pathogenesis of inflammatory bowel disease.” As discussed in that paper, T-cell response is pivotal in IBD pathogenesis. We have studied certain features of T-cell-mediated immunity in patients with IBD; therefore, I want to share our preliminary experience of immunological studies conducted on patients with IBD.
We aimed to study T-cell-mediated immune response against cytomegalovirus (CMV) in order to investigate the immunological characteristics of IBD and the difference between UC and CD for 2 reasons. One reason is the difference in the clinical importance of UC and CD, where CMV reactivation is mostly challenging in UC patients. The other reason is that most of the Korean population possesses the ability to mount a memory T-cell response against CMV due to past exposure to the virus. We suppose that the difference in immune response against CMV between UC and CD may explain the difference in the clinical importance of CMV reactivation between UC and CD.
We collected blood and colon mucosa samples from patients with UC, CD and healthy individuals. We isolated peripheral blood mononuclear cells (PBMCs) from CMV IgG-positive patients or healthy donors, who can mount a memory T-cell response against CMV infection due to previous virus exposure. Treatment-naïve patients or patients taking only 5-aminosalicylic acid were included in the final analysis due to the possible interfering effect of concurrent usage of immunosuppressants or biological agents on in vitro T-cell activation. PBMCs were stimulated with CMV antigen (i.e., CMV pp65 recombinant protein, PepTivator; Miltenyi Biotec, Bergisch Gladbach, Germany) and CD3/CD28 antibody (pan-memory T-cell stimulator that served as a positive control). Colonic mucosal tissue was collected by multiple biopsies and immune cells in lamina propria were extracted from the intestinal tissue homogenates. We assessed CMV antigen-stimulated or CD3/CD28 signal-induced cytokine production of T-cells by intracellular cytokine staining and flow cytometric analysis. We focused on Th1 cytokine interferon-γ (IFN-γ) and Th2 cytokine interleukin 4 (IL-4).
We collected blood samples from 22 patients and colon samples from 14 patients between June 2013 and December 2013; however, only a limited number of samples with adequate quantity and reliable immune response from immunosuppressive or biological agent-naïve patients were obtained. Finally, blood samples of 5 UC patients, 3 CD patients, and 5 healthy volunteers were included in this study. In contrast to blood samples, colon samples from IBD patients and healthy volunteers did not show any reliable cytokine production following stimulation of mucosal T-cells with CMV antigen or CD3/CD28 antibody. Nevertheless, we could clearly identify the mucosal T-cell population and classify the T cell subsets by flow cytometry.
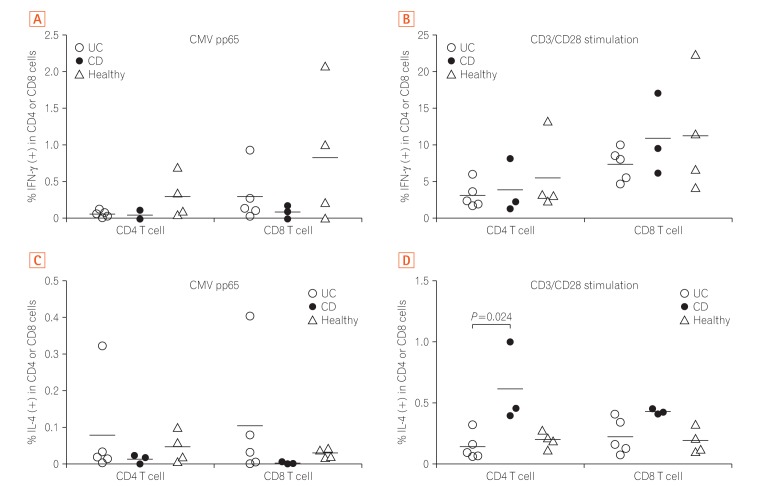
Fig. 1 shows the production of IFN-γ (Fig. 1A and B) and IL-4 (Fig. 1C and D) from peripheral blood memory T-cells stimulated with CMV-pp65 peptide or CD3/CD28 antibody. IFN-γ production against CMV was lower in UC and CD patients than in healthy donors (Fig. 1A). However, IFN-γ production after CD3/CD28 stimulation was comparable among the 3 groups (Fig. 1B). IL-4 production against CMV was higher in UC patients than in the other groups (Fig. 1C). Unexpectedly, IL-4 production from both CD4 and CD8 T-cells after CD3/CD28 stimulation was higher in CD patients than in the other groups (Fig. 1D). Although statistical calculation could be unreliable due to the small sample size, Kruskal-Wallis test for IL-4 production by CD4 T cells after CD3/CD28 stimulation revealed a P-value of 0.029 and post-hoc analysis revealed a significant difference between UC and CD (P=0.024) (Fig. 1D).
Taken together, the PBMC data suggested that the production of viral protective cytokine IFN-γ is decreased in IBD patients as compared to that of healthy donors when memory T-cells are stimulated with CMV peptide,2 while the production of IL-4 which can down-regulate antiviral activity34 seems to be increased in UC patients as compared to that of CD or healthy donors. This can be one clue explaining why CMV colitis is frequently encountered in UC patients in clinical practices.
For colonic mucosal T-cells, we could not stimulate cytokine production in vitro, but we could identify T-cell subsets. Interestingly, CD4 T-cells are significantly enriched in inflamed mucosa as compared to that of normal mucosa in the same UC patients, showing that CD4 T-cells play a critical role in UC pathogenesis, which is consistent with previous studies.5 Even though we failed to stimulate mucosal T-cell cytokine production by antigen presentation, we could stimulate massive cytokine production from mucosal T-cells by chemicals phorbol myristate acetate (PMA) and ionomycin which act directly on T-cells and do not need antigen presentation by antigen presenting cells. So, we suppose that we need to modify in vitro stimulation method of colonic mucosal T-cells by addition of professional antigen presenting cells (such as macrophage or dendritic cells) into the T-cell culture vial in order to get significant T-cell cytokine production after CMV antigen stimulation.
In conclusion, we could evaluate the T-cell composition and cytokine production from peripheral blood sample and could analyze T-cell composition from colon tissue sample. We could compare these characteristics among 3 different groups. Unfortunately, our study was prematurely terminated due to the time constraints and difficulty in obtaining blood and colon samples from adequate population. T-cell cytokine is important in IBD,6 so functional analysis of T-cells like this, would help identifying pathophysiology of IBD and finding new treatment.
Notes
References
1. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018; 16:26–42.

2. Biron CA, Tarrio ML. Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Med Microbiol Immunol. 2015; 204:345–354. PMID: 25850988.

3. Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996; 70:7103–7107. PMID: 8794356.

4. Gonzales-van Horn SR, Farrar JD. Interferon at the crossroads of allergy and viral infections. J Leukoc Biol. 2015; 98:185–194. PMID: 26026068.

5. Lu JT, Xu AT, Shen J, Ran ZH. Crosstalk between intestinal epithelial cell and adaptive immune cell in intestinal mucosal immunity. J Gastroenterol Hepatol. 2017; 32:975–980. PMID: 28072910.

6. Chen ML, Sundrud MS. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis. 2016; 22:1157–1167. PMID: 26863267.

Fig. 1
CD4 or CD8 cytokine production (interferon-γ [IFN-γ] and interleukin 4 [IL-4]) in response to cytomegalovirus (CMV) antigen or CD3/CD28 stimulation of UC, CD and healthy donor peripheral blood mononuclear cell. (A) IFN-γ production against CMV was lower in UC and CD patients than in healthy donors. (B) IFN-γ production after CD3/CD28 stimulation was comparable among the 3 groups. (C) IL-4 production against CMV was higher in UC patients than in the other groups. (D) IL-4 production from both CD4 and CD8 T-cells after CD3/CD28 stimulation was higher in CD patients than in the other groups.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download