Abstract
Background/Aims
Because of the similarities in the clinical presentations of Crohn's disease (CD) and intestinal tuberculosis (ITB), differential diagnosis is critical. Mesenteric adipose tissue hypertrophy and creeping fat are characteristic features of CD. The purpose of this study was to assess the usefulness of visceral fat for the differential diagnosis of CD and ITB.
Methods
We conducted a retrospective review of 50 patients with findings of CD or ITB between January 2005 and July 2008. Abdominal computed tomography (CT) was performed on all subjects during their first evaluation. The abdominal fat area was assessed using quantitative abdominal CT.
Results
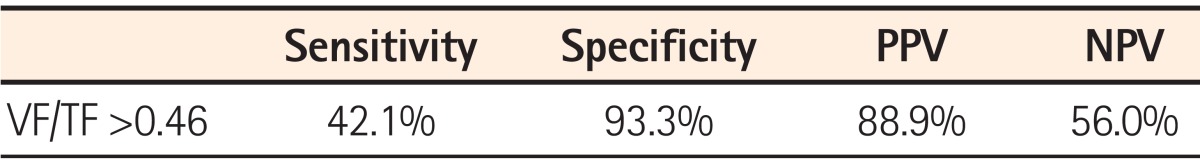
The ratio of visceral fat to total fat (VF/TF) was significantly higher in male CD patients than in male ITB patients. The ratio of visceral fat to subcutaneous fat (VF/SF) was also higher in CD patients than in patients with ITB. For a VF/TF cut-off value of 0.46, the sensitivity and specificity for the diagnosis of CD were 42.1% and 93.3% respectively, with positive and negative predictive values of 88.9% and 56.0%, respectively.
Intestinal tuberculosis (ITB) and CD are chronic granulomatous diseases of the intestine. Although these diseases have similar clinical manifestations, their ultimate courses and treatments are quite different. While ITB can be completely cured with the appropriate treatment, CD, in contrast, is not curable. In addition, many immunosuppressive agents that are very useful for the treatment of CD patients can be harmful to ITB patients. Therefore, the differential diagnosis of these two diseases is of extreme clinical importance.1
In developing countries, where both ITB and CD coexist, the differential diagnosis of these two conditions poses a challenge to clinicians. Furthermore, in both the developing and developed countries, there has been an increase in the number of cases of ITB due to the aquired immune deficiency syndrome (AIDS) epidemic, drug abuse, and influx of immigrants in recent years.2-5
Although a variety of clinical, endoscopic, and radiologic criteria have been introduced for the differential diagnosis of these two conditions, these have several limitations.6-13 Fat wrapping, defined as hypertrophied adipose tissue extending from the mesenteric attachment that partially covers the intestinal circumference, is common in both the small and large intestines and is considered a hallmark of CD.14,15 We hypothesized that mesenteric fat analysis can be used for the differential diagnosis of CD and ITB. The purpose of this study was to assess the usefulness of visceral fat (VF) measurements in the differential diagnosis of CD and ITB.
Between January 2005 and July 2008, 50 patients were given either a tentative or definite diagnosis of ITB or CD based on initial work-up from the results taken from clinical history, endoscopy, radiologic investigation, surgery, and pathologic evaluation at the Hanyang University Hospital, Seoul, Korea. All of these patients were included in this study. Abdominal CT was performed on all subjects at initial admission. Total abdominal fat (total fat [TF], VF, and subcutaneous fat [SF]) was assessed using quantitative abdominal CT (Fig. 1). In addition, we analyzed the clinical characteristics, laboratory findings, and endoscopic findings of all subjects. The study was approved by the institutional review board of our medical center.
The diagnosis of ITB was considered to be established when at least one of the following criteria was met: a) histologic evidence of caseating necrosis; b) histologic demonstration of acid-fast bacilli; c) growth of Mycobacterium tuberculosis on tissue culture; d) clinical, colonoscopic, radiologic, and/or operative evidence of TB elsewhere; e) response to antituberculous therapy without subsequent recurrence in patients with clinical, colonoscopic, radiologic, and/or operative evidence of ITB.16
A tentative diagnosis of CD was made if at least two of the following criteria were met: a) clinical history of abdominal pain, weight loss, malaise, diarrhea, and/or rectal bleeding; b) endoscopic findings of mucosal cobblestoning, linear ulceration, skip areas, or perianal disease; c) radiologic findings of stricture, fistula, mucosal cobblestoning, or ulceration; d) macroscopic appearance of bowel-wall induration, mesenteric lymphadenopathy, and creeping fat at laparotomy; e) pathologic findings of transmural inflammation and/or epithelioid granulomas.17
Body fat distribution was assessed using CT with a 10 mm thick slice at the level of the fourth lumbar vertebra. The technique used for fat tissue measurements in CT cross-sectional images has been standardized and validated.18 The VF area was defined as all of the pixels with adipose tissue attenuation coefficients. The tomographic attenuation of adipose tissue was defined to be between -150 and -50 Hounsfield units. The SF area was defined as the adipose area between the two defined contours, while the total abdominal fat area was defined as the sum of VF and SF (Fig. 2).
An Olympus CF-H260L and a CF-H260I video colonoscope (Olympus Optical Co., Ltd., Tokyo, Japan) were used. For bowel cleansing, patients were asked to drink either 4 L of a polyethyleneglycol-electrolyte solution (Colonlyte; Taejun, Seoul, Korea) or 90 mL of a sodium phosphate solution (Fleet; CB Fleet Company, Inc., Lynchburg, VA, USA). All procedures were performed using conscious sedation/analgesia with intravenous midazolam and pethidine titrated as required.
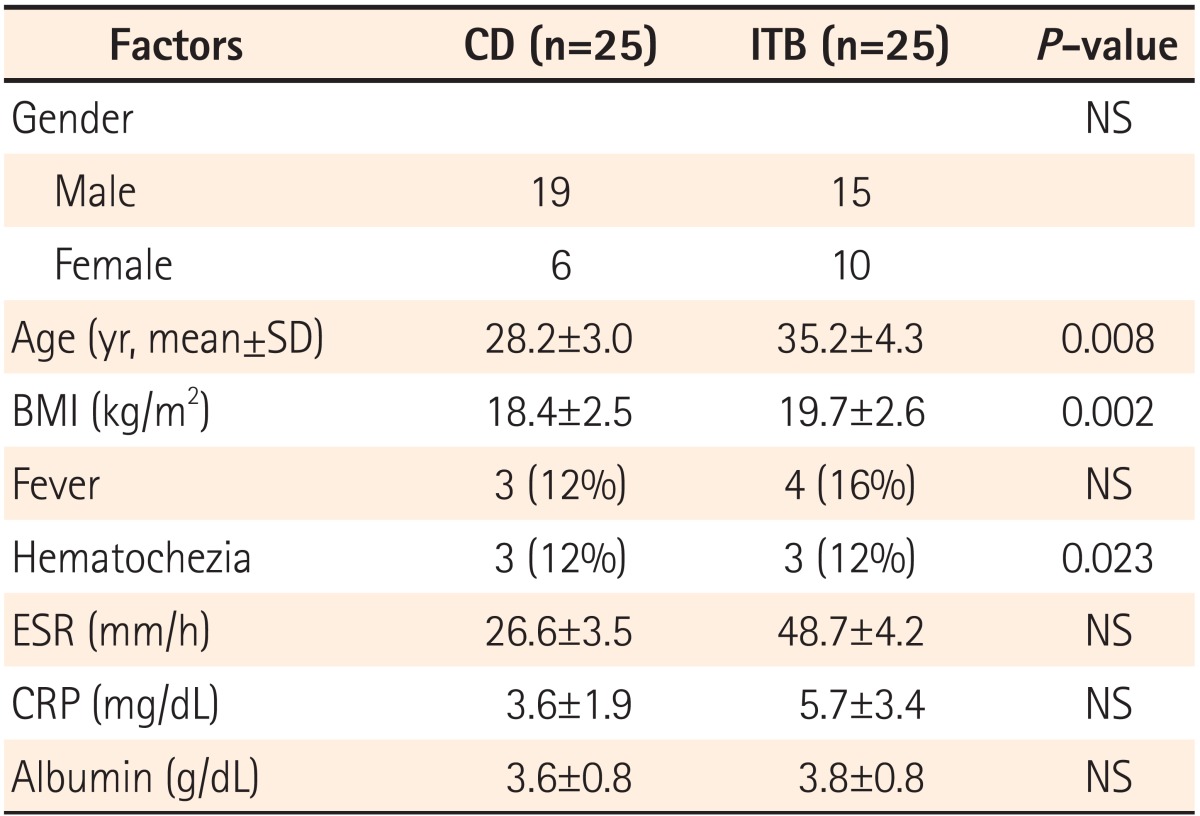
Twenty-five patients with CD (male:female, 19:6) and 25 patients with ITB (male:female, 15:10), were evaluated in this study. Results of the analysis of variance test for initial white blood cell count, ESR, CRP, or serum albumin levels revealed no significant difference between patient groups (Table 1).
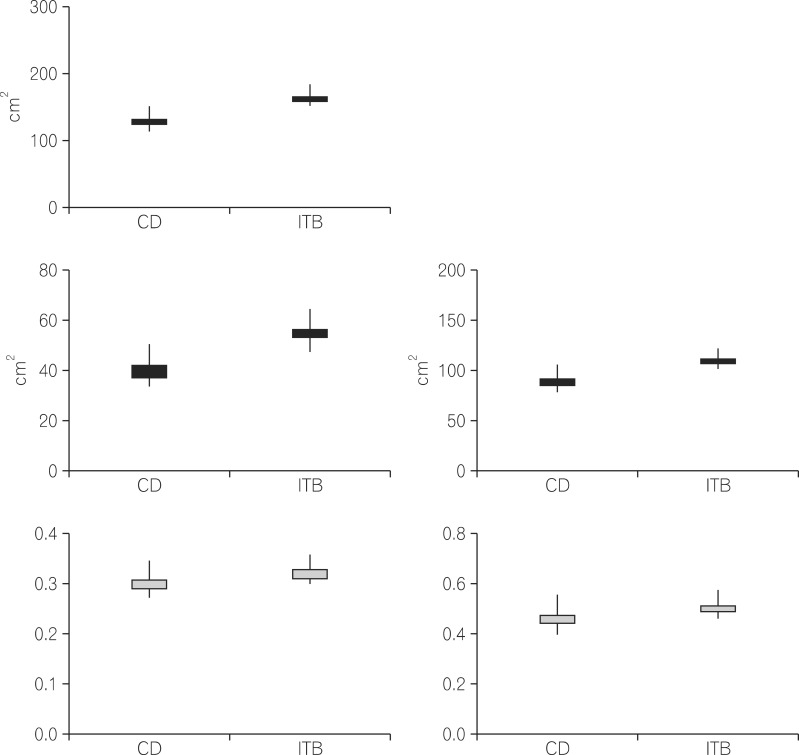
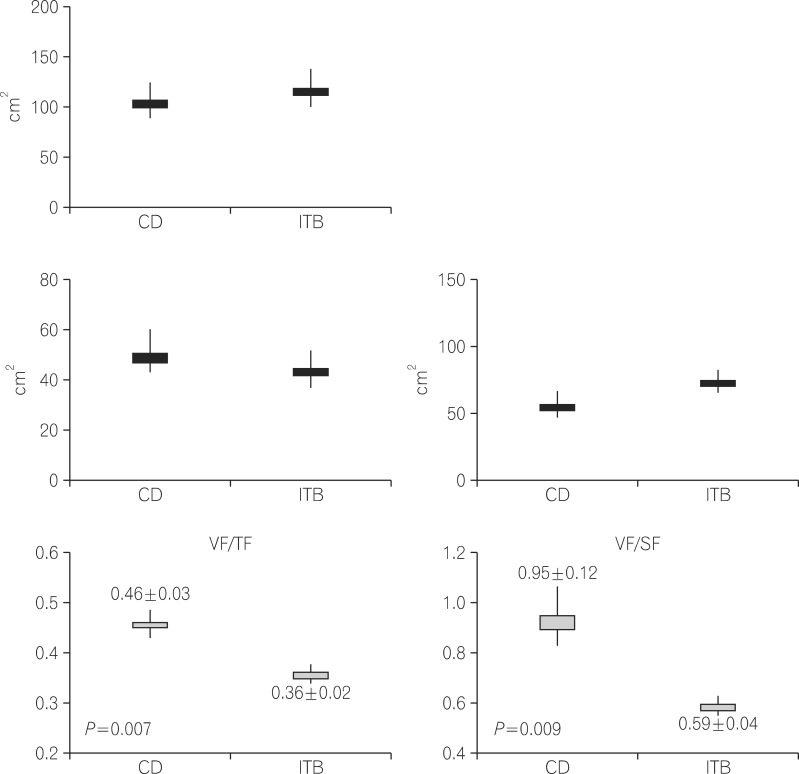
There were no significant differences in the amount of TF, VF, and SF among the female patients in the three groups. Furthermore, the ratio of VF/TF and the ratio of VF/SF were not significantly different among the female patients in the three groups. The abdominal fat area was similar among all female patients (Fig. 1). There were no differences in the amount of TF, VF, and SF among male patients in the three groups. However, the ratio of VF/TF was significantly higher in male CD than in male ITB patients (0.46±0.03 vs. 0.36±0.02, respectively; P=0.007). The ratio of VF/SF was also higher in male patients with CD compared to that in the other two groups (0.95±0.12 vs. 0.59±0.04 vs. 0.69±0.05, respectively; P=0.009) (Fig. 2).
VF area and various clinical parameters such as CDAI, ESR, CRP, and serum albumin showed no significant correlation in CD patients, as assessed using the Spearman correlation coefficients (r=0.084 for CDAI, r=0.296 for ESR, r=0.049 for CRP, r=0.109 for albumin) (P=0.57 for CDAI, P=0.46 for ESR, P=0.68 for CRP, P=0.76 for albumin).
Using a VF/TF cut-off value of 0.46, the sensitivity and specificity for the diagnosis of CD were 42.1% and 93.3%, respectively, and the positive and negative predictive values were 88.9% and 56.0%, respectively (Table 2).
To the best of our knowledge, this study is the first to investigate the clinical importance of VF in the differential diagnosis of CD and ITB. Because the use of steroids in CD could be catastrophic in the presence of active TB, the differential diagnosis of these two diseases is clinically important.19 However, the differential diagnosis of these two conditions is very difficult because of the difficulty involved in isolating M. tuberculosis and the considerable overlap in clinical presentation, endoscopic and radiological findings, and pathologic features. For this reason, many studies have proposed and evaluated differential diagnostic methods to distinguish between these conditions. A variety of clinical, endoscopic, and radiological criteria have been recommended for differential diagnosis, but these criteria have several limitations.20 In the United States, there has been a marked resurgence of TB in recent years in the wake of the AIDS epidemic and the large influx of immigrants.2,5 In Korea, the incidence of CD, which has previously been considered a rare disease, is rising.21 Therefore, differential diagnosis of TB and CD is important as these diseases often coexist both in the developing and developed countries.
Abdominal CT is more accurate and more reproducible than anthropometry for assessing body fat distribution. Abdominal fat comprises subcutaneous adipose tissue and visceral adipose tissue. Use of CT scanning allows the direct assessment of these two fat compartments, whereas anthropometric measurements do not. The measurement of VF surface area at the L4-5 level has been used extensively as an indicator of abdominal fat, and it has been shown in case control and cohort studies to be a strong predictor of insulin resistance syndrome, diabetes, and coronary artery disease.22,23
Transmural inflammation is the histological hallmark of CD. The macroscopic features of the mesentery adjacent to the intestinal segments involved in CD often include characteristics of hypertrophied adipose tissue such as fat wrapping and mesenteric thickening. Fat wrapping, defined as hypertrophied adipose tissue extending from the mesenteric attachment that partially covers the intestinal circumference, is common in both the small and large intestines and is considered a hallmark of CD. Yamamoto et al. suggested that adipocytes in hypertrophied mesenteric adipose tissue produce and secrete significant amounts of adiponectin, which could be involved in the regulation of intestinal inflammation associated with CD.15 Schaffleret et al. reported that CD patients treated with steroids had significantly lower vascular endothelial growth factor secretion rates compared to those in CD patients not receiving steroids. This study suggests that creeping fat is an important source of vascular endothelial growth factor secretion. Furthermore, mesenteric adipocytes or specific proteins secreted by mesenteric adipose tissue may play a role in the pathogenesis of CD.20 Therefore, mesenteric adipocytes may act not only as energy storage cells but also as immunoregulating cells in the case of intestinal inflammation via adiponectin production. The prevalence of fat abnormalities in CD has not been formally assessed by population-based studies. In a consecutive and unselected group of 27 intestinal resections performed on 25 patients with histologically-confirmed CD, fat-wrapping was identified in 12 of 16 ileal resections and in 7 of 11 large bowel resections.14 In contrast, TB is a catabolic infectious disease, thus patients with malnutrition and relatively thin patients are in the high-risk group. Additionally, ITB may have different CT findings from those of CD, such as pericecal and mesenteric fat stranding and regional lymphadenopathy.
Irrespective of BMI, CD patients accumulate intra-abdominal fat and have mesenteric obesity with the characteristic development of creeping fat.
The VF/SF ratio is used for the diagnosis of VF type obesity. VF is metabolically active and is strongly associated with elevated serum levels of several proinflammatory cytokines such as IL-6 and tumour necrosis factor α. Creeping fat is a characteristic feature of CD, and adipose tissue secretes adipocytokines and chemokines/growth factors such as vascular endothelial growth factor. Some studies reported that a high ratio of VF/SF area is a marker of aggressive CD.24
Although attempts have been made to distinguish between CD and ITB, no specific differential diagnostic method exists. The diagnostic values of other investigative modalities, such as pathologic evaluation, polymerase chain reaction, and endoscopic differentiation, have been investigated. Pulimood et al. reported that the type and frequency of granulomas, the presence or absence of ulcers lined by epithelioid histiocytes and microgranulomas, and the distribution of chronic inflammation could be used as histologic parameters to differentiate between CD and ITB.6 Gan et al. reported that a polymerase chain reaction assay was useful for rapid and accurate differential diagnosis of these two conditions.12 Lee et al. suggested that a systematic analysis of colonoscopic findings was very useful for the differential diagnosis of these two diseases.1
However, the diagnostic methods mentioned above are invasive and/or expensive. In our study, the VF/TF and VF/SF ratios were significantly different between CD and ITB male patients. For a VF/TF cut-off value of 0.46, the specificity for diagnosis of CD was 93.3% with a positive predictive value of 88.9%. This result suggests that the area of VF can be used as a diagnostic parameter for differentiating between CD and ITB, especially in males. Why different results were obtained for females and males is unclear. One possible explanation for this is that women tend to accumulate less VF with weight gain compared with men.
This study had several limitations. First, the patient populations were very small; larger-scale studies should be performed in the future to confirm our findings. Second, we only analyzed the VF area at the level of the fourth lumbar vertebra, irrespective of bowel inflammatory lesions. A systematic analysis of fat around bowel inflammatory lesions is also needed. Third, the number of women with CD was small; this was a limitation in the analysis of the correlation between VF and CD.
In conclusion, we suggest that creeping fat analysis using abdominal CT can be a simple, non-invasive, and economic method to differentiate between CD and ITB. In the future, more large-scale studies are needed.
References
1. Lee YJ, Yang SK, Byeon JS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn's disease. Endoscopy. 2006; 38:592–597. PMID: 16673312.

2. Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol. 1998; 93:692–696. PMID: 9625110.

3. Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993; 88:989–999. PMID: 8317433.
5. Rieder HL, Cauthen GM, Kelly GD, et al. Tuberculosis in the United States. JAMA. 1989; 262:385–389. PMID: 2661873.

6. Pulimood AB, Ramakrishua BS, Kurian G, et al. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn's disease from tuberculosis. Gut. 1999; 45:537–541. PMID: 10486361.

7. Das P, Shukla HA. Clinical diagnosis of abdominal tuberculosis. Br J Surg. 1976; 63:941–946. PMID: 1009343.

8. Segal I. Intestinal tuberculosis, Crohn's disease and ulcerative colitis in an urban black populations. S Afr Med J. 1984; 65:37–44. PMID: 6695246.
9. Burke GJ, Zafar SA. Problems in distinguishing tuberculosis of the bowel from Crohn's disease in Asians. Br Med J. 1975; 4:395–397. PMID: 1192087.

10. Aoki G, Nagasako K, Nakae Y, Suzuki H, Endo M, Takemoto T. The fibercolonoscopic diagnosis of intestinal tuberculosis. Endoscopy. 1975; 7:113–121.

11. Tandon HD, Prakash A. Pathology of intestinal tuberculosis, and its distinction from Crohn's disease. Gut. 1972; 13:260–269. PMID: 5033841.

12. Gan HT, Chen YQ, Ouyang Q, Bu H, Yang XY. Differentiation between intestinal tuberculosis and Crohn's disease in endoscopic biopsy specimens by polymerase chain reaction. Am J Gastroenterol. 2002; 97:1446–1451. PMID: 12094863.

13. Pulimood AB, Peter S, Ramakrishna B, et al. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn's disease. J Gastroenterol Hepatol. 2005; 20:688–696. PMID: 15853980.
14. Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's diseases: pathological basis and relevance to surgical practice. Br J Surg. 1992; 79:955–958. PMID: 1422768.

15. Yamamoto K, Kiyohara T, Murayama Y, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005; 54:789–796. PMID: 15888786.

16. Kim YS, Kim YH, Lee KM, Kim JS, Park YS. IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Diagnostic guideline of intestinal tuberculosis. Korean J Gastroenterol. 2009; 53:177–186. PMID: 19835219.
17. Ye BD, Jang BI, Jeen YT, Lee KM, Kim JS, Yang SK. IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Diagnostic guideline of Crohn's disease. Korean J Gastroenterol. 2009; 53:161–176. PMID: 19835218.
18. Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: A prospective study among Japanese Americans. Diabetes Care. 2000; 23:465–471. PMID: 10857936.
19. Makanjuola D. Is it Crohn's disease or intestinal tuberculosis? CT analysis. Eur J Radiol. 1998; 28:55–61. PMID: 9717624.

20. Schaffler A, Furst A, Buchler C, et al. Vascular endothelial growth factor secretion from mesenteric adipose tissue and from creeping fat in Crohn's disease. J Gastroenterol Hepatol. 2006; 21:1419–1423. PMID: 16911686.

21. Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010; 4:1–14. PMID: 20479907.

22. El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett's esophagus. Am J Gastroenterol. 2005; 100:2151–2156. PMID: 16181362.

23. Giovannucci E. Obesity, gender, and colon cancer. Gut. 2002; 51:147. PMID: 12117867.
24. Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn's disease. Clinical Gastroenterology and Hepatology. 2011; 9:684–687. PMID: 21642015.

Fig. 1
Abdominal fat in female patients. (A) Total fat (TF) (P=0.144). (B) Visceral fat (VF) (P=0.360). (C) Subcutaneous fat (SF) (P=0.112). (D) VF/TF (P=0.911). (E) VF/SF (P=0.814). ITB, intestinal tuberculosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download