Abstract
Objectives
Acquired immunodeficiency syndrome (AIDS) is a disease of the human immune system caused by the human immunodeficiency virus (HIV). People with AIDS are much more vulnerable to infections, including opportunistic infections and tumors, than people with a healthy immune system. The objective of this study was to correlate oral lesions associated with HIV/AIDS and immunosuppression levels by measuring clusters of differentiation 4 (CD4) cell counts among patients living in the middle western regions of Ghana.
Materials and Methods
A total of 120 patients who visited the HIV clinic at the Komfo Anokye Teaching Hospital and the Regional Hospital Sunyani of Ghana were consecutively enrolled in this prospective and cross-sectional study. Referred patients' baseline CD4 counts were obtained from medical records and each patient received an initial physician assessment. Intraoral diagnoses were based on the classification and diagnostic criteria of the EEC Clearinghouse, 1993. After the initial assessment, extra- and intraoral tissues from each enrolled patient were examined. Data analyses were carried out using simple proportions, frequencies and chi-square tests of significance.
Results
Our study included 120 patients, and was comprised of 42 (35.0%) males and 78 (65.0%) females, ranging in age from 21 to 67 years with sex-specific mean ages of 39.31 years (males) and 39.28 years (females). Patient CD4 count values ranged from 3 to 985 cells/mL with a mean baseline CD4 count of 291.29 cells/mL for males and 325.92 cells/mL for females. The mean baseline CD4 count for the entire sample was 313.80 cells/mL. Of the 120 patients we examined, 99 (82.5%) were observed to have at least one HIV-associated intraoral lesion while 21 (17.5%) had no intraoral lesions. Oral candidiasis, periodontitis, melanotic hyperpigmentation, gingivitis and xerostomia were the most common oral lesions.
The human immunodeficiency virus (HIV) targets and infects immune cells; acquired immunodeficiency syndrome (AIDS) is a disease of the human immune system that is caused by HIV. Because HIV interferes with the immune system, people with AIDS are much more likely to contract infections that do not affect people with healthy immune systems, including opportunistic infections and tumors. HIV has many transmission routes, including sexual contact (anal, vaginal or oral), blood transfusion, shared hypodermic needles, and vertical transmission through breastfeeding. HIV is a major health problem in many populations and is considered to be a pandemic. The national HIV prevalence in Ghana in 2013 was estimated to be 1.3%; this indicates that an estimated 224,488 people in Ghana have HIV/AIDS, including 189,931 adults and 34,557 children1.
The cluster of differentiation 4 (CD4) cell count is more sensitive to sudden changes in a person's immunity and, thus, is a better indicator of HIV/AIDS progression2. CD4 counts can differ among individuals, depending on their age, sex and immune status. Oral lesions are generally an early sign of HIV infection, but could also be used to predict the progression of HIV/AIDS in patients3. For example, in 2011, one study reported that during diagnosis of AIDS-related oral lesions identified in an untreated HIV-positive patient, highly active antiretroviral therapy (HAART) was initiated and this approach could also be applied in HIV/AIDS patients with a CD4 count higher than 350 cells/mL4.
The goal of this study was to increase knowledge about this critical clinical decision and to confirm whether oral manifestations in Ghanaian HIV-positive patients are useful for determining disease progression.
Our samples came from the HIV Clinic at the Komfo Anokye Teaching Hospital (KATH) and the Regional Hospital Sunyani (RHS) in the Brong Ahafo region of Ghana, which is under the Directorate of Medicine of the hospital and serves as the referral center for Ghana's Northern sector during August of 2014 to March of 2016. Although the clinic provided both out- and in-patient care, we only enrolled outpatient cases from the HIV-HAART clinic and the maxillofacial dental clinic. We enrolled all HIV/AIDS-positive patients that had not yet started HAART and we excluded all patients with a previous history of receiving HAART.
The examiner, received training on our diagnostic criteria prior to conducting patient examinations. The initial or baseline CD4 count from new patients and recruited AIDS patients was retrieved from medical records and other relevant documentation. We also administered a well-structured questionnaire to collect relevant social and demographic details from the study patients. None of the participants declined participation, resulting in a 100% participation rate.
Convenience sampling was used. All adult patients living with HIV/AIDS (PLWHA) who attended the clinic and satisfied our inclusion criteria were recruited over a three-week period; recruitment stopped once we obtained the desired sample size. A total of 120 patients were enrolled from the out-patient department of the HIV and maxillofacial clinic at KATH and RHS. All PLWHA were initially referred to after receiving a confirmatory Western blot technique test result; once their CD4+ count was determined, a clinic physician established a management protocol according to the World Health Organization (WHO) clinical staging criteria. Initial patient assessments and categorization were also completed by a clinic physician and in accordance with the WHO. Dental examinations were conducted by the principal investigator and performed on all PLWHAs; all dental examinations were performed under a headlamp on all consenting PLWHA while sitting upright in a dental chair with a dental mirror, wooden spatula and a dental probe. Oral examinations were regularly performed with well-trained dental assistants.
This study was approved by the Committee on Human Research, Publications and Ethics of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology in Kumasi. Permission to carry out the study was granted by the head of the Directorate of Medicine, who runs the HIV clinic. The study was registered with the Research and Development Unit of KATH and given a certificate of registration. We sought written consent from each participant and ensured that all data would be handled with complete confidentiality. PLWHA who were found to have dental lesions, such as caries, periodontal disease, or missing teeth, were referred to as the appropriate unit of the RHS clinic for management. The data were processed using the SPSS statistical software ver. 16.0 (SPSS Inc., Chicago, IL, USA). Quantitative data analyses consisted of simple proportions, frequency and chi-square tests. The mean values are followed by 95% confidence intervals.
A total of 120 patients participated in this study, including 42 males (35.0%) and 78 females (65.0%) with a ratio of 1:1.86. Patient ages ranged from 21 to 67 years with a mean age of 39.31 years for males and 39.28 years for females. Age in males varied by a range of 36 years, while female patient ages varied by 46 years. The age distribution is shown in Table 1.
Patient CD4 count values ranged from 3 to 985 cells/mL with a mean baseline CD4 count of 291.29 cells/mL for males and 325.92 cells/mL for females. The mean CD4 count for the entire sample was 313.80 cells/mL. About three-quarters of the patients (75.4%) were classified as having a WHO clinical disease stage I or II.
Ninety-nine patients (82.5%) were observed to have at least one HIV-associated oral lesion, while 21 patients (17.5%) had no oral lesions. Some patients had more than one oral lesion. We report all observed oral lesions in descending order of occurrence in Table 2. The distribution of oral lesions by sex is also shown in Table 2.
The most common lesion was oral candidiasis, which was observed in 47 patients (39.2%). We also recorded other forms of oral candidiasis, such as pseudomembranous, hyperplastic, erythematous, and median rhomboid glossitis. In addition to oral candidiasis lesions, we recorded periodontitis in 45 patients (37.5%) and four cases of periodontal disease, including linear gingival erythema, necrotizing ulcerative gingivitis (NUG), necrotizing ulcerative periodontitis (NUP), and necrotizing stomatitis. Twenty-five patients (20.8%) had melanotic hyperpigmentation, 23 patients (19.2%) had gingivitis, and 21 patients (17.5%) had xerostomia. We classified periodontitis as both localized and generalized periodontal disease, and patients with xerostomia were screened for any clinically active on-going processes in the major salivary glands to ensure they did not have enlargements, tenderness or induration.
Ten patients (8.3%) had oral hairy leukoplakia (OHL), which appeared as thick white patches that did not wipe away or as vertical corrugations with a hair-like appearance. These lesions were usually found on the lateral margins of the tongue, inner cheek, or lower lip. They are asymptomatic and often associated with oral candidiasis. Five patients (4.2%) had herpes simplex virus (HSV) outbreaks that appeared as a crop of vesicles on the hard palate or on the vermilion borders of the lips and perioral skin. Four patients (3.3%) had angular cheilitis and two patients (1.7%) had an aphthous ulcer.
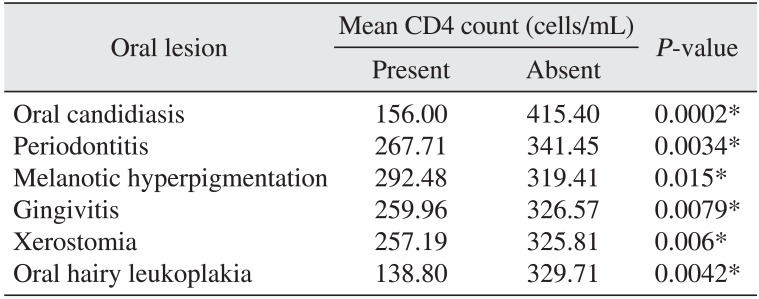
Six of the nine types of oral lesions that we examined, including oral candidiasis, periodontitis, melanotic hyperpigmentation, gingivitis, xerostomia, and OHL, were significantly identified in oral lesion patients and were not identified in patients that did not have lesions.(Table 3) These conditions were also significantly associated with declining baseline CD4 counts from 292.48 cells/mL down to 138.80 cells/mL; OHL was associated with lower CD4 counts and melanotic hyperpigmentation was identified in patients with higher count ranges.(Fig. 1)
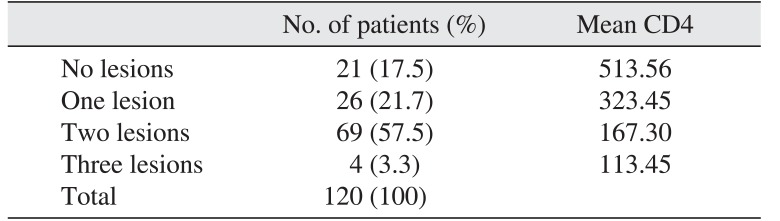
Some patients had more than one variant: 69 patients (57.5%) had two lesions, four patients (3.3%) had three lesions, and 26 patients (21.7%) had only one lesion. Most patients had two oral lesions. The mean baseline CD4 count of patients with two lesions was 167.30 cells/mL, the mean baseline CD4 count for patients with three lesions was 113.45 cells/mL, patients with one lesion had a mean baseline count of 323.45 cells/mL, and those without lesions had a count of 513.56 cells/mL.(Table 4)
Although there have been reports of delayed antibody response to HIV1 infection, 95% or more of infected people develop detectable antibodies to HIV1 within 3 to 6 months. The interval between infection and seroconversion or detection of HIV-specific antibodies has been called the window period5. Standard screening tests for HIV infection include enzyme-linked immunoassay, Western blot, p24 capture assay, and the HIV RNA detection method. We used the Western blot method, which is the most commonly used confirmatory test because it targets three of the major HIV genes, offering conclusive evidence of HIV infection56.
A known characteristic of HIV/AIDS pathogenesis is the targeting of human immune cells that bear the CD4 surface marker. HIV infections cause a gradual decrease in the number of CD4+ cells, the most important being the T-helper cells (CD4 T-cells), B lymphocytes, macrophages, and natural killer cells. The CD4 cell count (the amount of CD4 lymphocyte cells per milliliter) and viral RNA load (quantity of HIV-1 RNA copies per milliliter) are currently the most reliable laboratory indicators of HIV progression. HIV-positive patients with CD4 lymphocyte-cell counts <200 per milliliter are considered severely immune depressed and HIV-positive patients with viral RNA loads >10,000 copies per milliliter are considered to have active viremia5678. CD4 cells coordinate a number of immunological functions and, as cell numbers decrease, the risk and severity of opportunistic infections increases and these opportunistic infections can become life-threatening. Previous research has indicated that the absolute number of CD4 lymphocytes in HIV-infected people falls from a normal level of 800-900 to 60-100 cells/mL within a year9. Because the loss of immune function is associated with declining CD4 counts, the Centers for Disease Control and Prevention stratify patients with HIV infection into three categories, based on CD4 counts: CD4 >500 cells/mL, 200–499 cells/mL, and <200 cells/mL, the lowest of which is associated with severe immune suppression and a fulfills the criteria for a disease that has progressed to AIDS510.
The age distribution of our subjects revealed a higher prevalence of HIV among people in their second (23.3%) and third (34.2%) decades, which is consistent with other studies111213. This finding also supports the UNAIDS/WHO report14 that about one-third of people currently living with HIV/AIDS are between 15 and 24 years old. The sex distribution of the sample population was similar to the national sex distribution in Ghana1 as well as that of other African countries, including Uganda, the Central African Republic, Equatorial Guinea and Gabon1115. These sex distributions are different from those in the USA, United Kingdom, Europe and other developed countries16; the difference in HIV sex distribution may be a result of the more pervasive acceptance of homosexuality in developed countries compared with Africa, whereas reduced socioeconomic opportunities, polygamy and unfavorable cultural practices in Africa, including increased vulnerability of women, may lead to a greater impact of heterosexual transmission and HIV prevalence14.
There are two main classification systems of oral lesions associated with HIV infection. The first is based on the etiology of the oral lesions. According to this system, oral lesions are classified as bacterial, viral, or fungal infections, as neoplastic, or as having another etiology. The more widely-used second system is based on the classification and diagnostic criteria developed in 1993 by EEC Clearinghouse's Oral Problems Related to HIV Infection1718, as well as by the UNAIDS/WHO19. This system classifies oral lesions into three groups according to their degree of association with HIV infection (i.e., strongly associated, less commonly associated, not associated)1718. We compared oral symptoms, including candidiasis, OHL, and periodontal disease (gingivitis and periodontitis) to symptoms classified as strongly associated by the EEC Clearinghouse and WHO. We also examined oral lesions, such as melanotic pigmentation, xerostomic salivary gland disease, and HSV, which are classified as less-commonly associated. Finally, we also included recurrent aphthous stomatitis, also known as aphthous ulcers, as a lesion of interest. In total, we examined six different types of oral diseases and their association with CD4 counts.
We found that 82.5% of our study sample had at least one type of oral lesion at the time of examination. The clinical oral lesions observed in this study have been discussed in the literature to varying degrees in different populations5202122232425.
Oral candidiasis was the most common specific oral lesion, and this finding is similar to many other studies252627. The prevalence may depend on the study population, diagnostic criteria, study design, and availability of HAART. Oral candidiasis is known to be a significant predictor of HIV progression in both adults and children, which is consistent with our results. The median survival time from clinical diagnosis to death is 3.4 years in HIV-infected children. The primary etiology for oral candidiasis is the fungus Candida albicans, although other Candida species may be involved. Oral candidiasis is often observed in one of four clinical forms. The erythematous type, also called atrophic candidiasis, appears clinically as multiple small or large patches and is most often localized to the tongue and/or palate. Pseudomembranous candidiasis, also known as oral thrush, is characterized by the presence of multiple superficial creamy white plaques that can be easily wiped off, revealing an erythematous base. They are usually located on the buccal mucosa, oropharynx, and/or dorsal face of the tongue. Hyperplastic candidiasis appears white and hyperplastic and cannot be removed by scraping. This form of oral candidiasis is rare in HIV-infected individuals. Angular cheilitis is characterized by the presence of erythematous fissures at the corners of the mouth. It is usually accompanied by another form of intraoral candidiasis2829. Treatment with topical and systemic antifungal agents is recommended.
OHL is more common among HIV-infected adults than among HIV-infected children. The reported prevalence of OHL in adults is 20% to 25%, increasing as CD4+ counts decrease; in children the prevalence is 2% to 3%. The presence of OHL is a sign of severe immunosuppression. OHL is a significant predictor of HIV disease progression in adults. Although its etiology is not clear, it seems to be caused by an Epstein-Barr virus infection. OHL presents as white, thick patches that do not wipe away and that may exhibit vertical corrugations with a hair-like appearance. The lesions usually start on the lateral margins of the tongue or sometimes inside the cheeks and lower lip. The lesions may be unilateral or bilateral, are usually asymptomatic, and often associated with oral candidiasis. OHL does not usually require treatment, except for severe cases for which systemic antivirals are recommended. Therapeutic management of oral candidiasis is required when OHL is associated with oral candidiasis.
HIV-associated periodontal disease is common among HIV-infected patients. It is characterized by bleeding gums, bad breath, pain, discomfort, mobile teeth, and, occasionally, sores. Its reported prevalence ranges widely, between 0% and 50%. HIV-associated periodontal disease can progress to life-threatening infections if left untreated, including Ludwig's angina and cancrum oris (noma). The periodontal and gingival diseases that we observed in this study population were generally severe. The prevalence of periodontitis (37.5%) and gingivitis (19.2%) were high compared with those reported in other studies: Adurogbangba et al.23 found periodontitis and gingivitis prevalence's of 2.7% and 4.3%, respectively, while Adedigba et al.25 found prevalences of 4.4% and 5.3%, respectively, in Nigeria. The Brong Ahafo region is a northern rural region of Ghana, with low socioeconomic conditions. Four types of HIV-associated periodontal disease have been described and identified in this region, including linear gingival erythema, NUG, NUP, and necrotizing stomatitis.
Our clinical classification for gingivitis included 1) Linear gingival hyperplasia, which is characterized by the presence of a 2-3 mm red band along the marginal gingiva and is associated with diffuse erythema on the attached gingiva and oral mucosa. 2) NUG is the ulceration, sloughing, or necrosis of one or more interdental papillae and is accompanied by pain, bleeding, and fetid halitosis. NUG is more common in adults than children. The degree of erythema is typically disproportionately intense compared with the amount of plaque present on the teeth. 3) NUP is characterized by extensive and rapid loss of soft tissue and teeth, and the most severe form, necrotizing stomatitis, involves acute and painful ulceronecrotic lesions on the oral mucosa that expose the underlying alveolar bone. Management and control of HIV-associated periodontal disease begins with good daily oral hygiene, including brushing, flossing, and use of mouthwash solutions, which are effective for preventing and controlling periodontal disease.
HSV infections may be either primary herpetic gingivostomatitis infections or secondary infections from herpes labialis. The prevalence of oral HSV infections varies between 10% and 35% in HIV-infected adults and children. An HSV outbreak that lasts longer than 1 month can indicate an underlying AIDS infection. HSV infections appear as a crop of vesicles usually localized on the keratinized hard palate or the gingival mucosa and/or vermilion borders of the lips and perioral skin. The vesicles rupture and form irregular painful ulcers which can interfere with mastication and swallowing, resulting in decreased oral intake and dehydration. Systemic therapy with antiviral agents is recommended, and treatment is more effective if it is instituted in the prodromal stage of infection.
Recurrent aphthous ulcers (RAUs) occur in about 1% to 7% of HIV-infected patients. They are painful ulcers that appear on the nonkeratinized oral mucosa, such as the labial and buccal mucosa, soft palate, and the ventral aspect of the tongue. Severe recurrent aphthous lesions usually occur when CD4+ counts are <100 cell/mL and may be suggestive of HIV disease progression. The etiology of RAUs is not well known, and RAUs may present as minor, major, or herpetiform aphthae. Minor aphthous ulcers are less than 5 mm in diameter and are covered by a pseudomembrane that is surrounded by an erythematous halo. The ulcers usually heal spontaneously without scarring. Major aphthous ulcers resemble minor aphthous ulcers, but they are fewer and larger in diameter (1–3 cm), are more painful, and may persist longer. Their presence interferes with mastication, swallowing, and speaking. Healing occurs over 2 to 6 weeks and scarring is common. Herpetiform aphthous ulcers occur as a crop of many small lesions that are 1 to 2 mm in diameter, which disseminate along the soft palate, tonsils, tongue, and/or buccal mucosa. The first line of RAU management is pain control and prevention of superinfection. Depending on the severity of the ulcers, topical and/or systemic steroid agents are recommended.
Melanotic hyperpigmentation was another commonly observed lesion in this study. It is well known that zidovudine, which is part of the antiretroviral therapy (ART) regime, can induce melanotic changes in the oral mucosa. None of our study subjects were on HAART, so the high prevalence of melanotic hyperpigmentation that we observed was independent of HAART or ART regimens.
We observed that lesion frequency in outpatients was significantly correlated with declining baseline CD4 counts, ranging from 290 to 140 cells/mL. This is consistent with the conclusion that, specific clinical presentations appear as immunity declines in Ghanaian PLWHA patients. This pattern is probably comparable to presentation patterns in other HIV-prevalent populations. Based on the results of this study, oral lesions could provide an opportunity for early clinical diagnoses because of the underlying association of CD4 cell counts; such clinical tools are particularly important in developing and low socioeconomic clinical environments. The results, presented Fig. 1, are similar to those from the Multicenter AIDS Cohort (MAC) study2030, which also compared the approximate timing of symptom presentation and CD4 cell counts. The MAC study explored general systemic presentations and found similar timings associated with CD4 declines, although they did not thoroughly explore oral lesion presentation.(Table 3)
The six oral lesions that were significantly correlated with CD4 cell counts were oral candidiasis, periodontitis, melanotic hyperpigmentation, gingivitis, xerostomia, and hairy leukoplakia.(Table 3) The relationship between lesion presentation and cell count was significant for cell-count ranges between 138.80 cells/mL and 292.48 cells/mL, with OHL being most common at the lowest end of the cell count range and melanotic hyperpigmentation at the highest. None of the patients examined were on HAART. Nevertheless, it is important to note that some were on at least one type of medication at the time of examination, such as antifungals, antibiotics, antituberculosis or hematinic therapy to treat opportunistic infections. Therefore, it is possible that these medications could have masked or affected the presence of any of these manifestations. We observed no cases of herpes zoster infection in this study, which is contrary to similar studies22 that reported high levels of herpes zoster. We also saw no cases of Kaposi's sarcoma, which is consistent with findings from India 202131, but not Zimbabwe24, where Kaposi's sarcoma was most common. One study reported that the CD4 count was 9 to 13 cells/mL in one Korean patient with Kaposi's sarcoma32.
Unfortunately, we did not have follow-up CD4 counts nor viral load data that was based on ART treatment progression because most of the patients live in rural settlements and do not complete consistent follow-up after the first visit or after recovery. One of the limitations to this study was that because follow-up was limited and inconsistent, the results are not compatible with current HIV treatment guidelines of CD4 testing every 3 to 6 months after first starting ART.
We found an oral lesion prevalence of 82.5% among our HIV-positive study subjects. Of the twelve clinical lesion types studied, six had a statistically significant association with declining CD4 baseline levels from 292.48 to 138.80 cells/mL; the pattern of decline had a clear cumulative appearance associated with specific oral lesions in our subjects. The mean CD4+ T-lymphocyte cell count for HIV-positive patients was 325 cells/mL. Low CD4+ levels were associated with greater prevalence of oral candidiasis, OHL, and periodontal diseases, indicating that these lesions could be a useful predictor of lower CD4 counts and HIV progression.
Because the current national HIV prevalence in Ghana is 1.3%1, our 120-person sample is not a true reflection of the prevalence of oral lesions in HIV-positive patients. We recommend further studies with a large sample size to better understand the association between virus status and the presence of oral lesions in PLWHA in Ghana and other sub-Saharan African countries. Finally, additional HIV awareness training for public awareness and education is needed in the African region, including Ghana.
References
1. Summary of the 2013 HIV Sentinel survey report [Internet]. Accra, Ghana: Ghana AIDS Commission;cited 2016 Mar 12. Available from: http://www.ghanaids.gov.gh/gac1/aids_info.php.
2. Parathyras J, Gebhardt S, Hillermann-Rebello R, Grobbelaar N, Venter M, Warnich L. A pharmacogenetic study of CD4 recovery in response to HIV antiretroviral therapy in two South African population groups. J Hum Genet. 2009; 54:261–265. PMID: 19282874.

3. Begg MD, Lamster IB, Panageas KS, Mitchell-Lewis D, Phelan JA, Grbic JT. A prospective study of oral lesions and their predictive value for progression of HIV disease. Oral Dis. 1997; 3:176–183. PMID: 9467362.

4. Cheng R, Patel S, Mandel L. Oral manifestations in untreated HIV patient. N Y State Dent J. 2011; 77:58–60. PMID: 22029118.
5. Saini R. Oral lesions: a true clinical indicator in human immunodeficiency virus. J Nat Sci Biol Med. 2011; 2:145–150. PMID: 22346226.

6. Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997; 126:946–954. PMID: 9182471.

7. Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996; 272:1167–1170. PMID: 8638160.

8. Vlahov D, Graham N, Hoover D, Flynn C, Bartlett JG, Margolick JB, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. JAMA. 1998; 279:35–40. PMID: 9424041.
9. Chan RK. Early clinical manifestations of HIV infection. Singapore Med J. 1990; 31:477–479. PMID: 2259948.
10. Beverly P, Helbert M. Immunology of aids; ABC of AIDS. 4th ed. New York: BMJ Publication Group;1997. p. 11–12.
11. Berkley S, Naamara W, Okware S, Downing R, Konde-Lule J, Wawer M, et al. AIDS and HIV infection in Uganda--are more women infected than men? AIDS. 1990; 4:1237–1242. PMID: 2088401.

12. Guteta S, Feleke Y, Fekade D, Neway M, Diro E. Prevalence of oral and perioral manifestations in HIV positive adults at Tikur Anbessa Teaching Hospital Addis Ababa, Ethiopia. Ethiop Med J. 2008; 46:349–357. PMID: 19271399.
13. Gasparin AB, Ferreira FV, Danesi CC, Mendoza-Sassi RA, Silveira J, Martinez AM, et al. Prevalence of oral lesions in persons with HIV and associated factors in a southern Brazilian city. Cad Saude Publica. 2009; 25:1307–1315. PMID: 19503961.
14. 2014 progress report on the global plan [Internet]. Geneva, Switzerland: UNAIDS;2014. 11. 11. cited 2017 Feb 9. Available from: http://www.unaids.org/en/resources/documents/2014/JC2681_2014-Global-Plan-progress.
15. Butt FM, Chindia ML, Vaghela VP, Mandalia K. Oral manifestations of HIV/AIDS in a Kenyan provincial hospital. East Afr Med J. 2001; 78:398–401. PMID: 11921559.
16. Greenwood I, Zakrzewska JM, Robinson PG. Changes in the prevalence of HIV-associated mucosal disease at a dedicated clinic over 7 years. Oral Dis. 2002; 8:90–94. PMID: 11991309.
17. EC Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Human Immunodeficiency Virus. Classification and diagnostic criteria for oral lesions in HIV infection. J Oral Pathol Med. 1991; 20:97–100. PMID: 1645406.
18. Classification and diagnostic criteria for oral lesions in HIV infection. EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. J Oral Pathol Med. 1993; 22:289–291. PMID: 8229864.
19. Ending the AIDS epidemic [Internet]. Geneva, Switzerland: UNAIDS;2014. 12. 01. cited 2017 Feb 9. Available from: http://www.unaids.org/en/resources/documents/2014/20141201_Paris_declaration.
20. Ranganathan K, Reddy BV, Kumarasamy N, Solomon S, Viswanathan R, Johnson NW. Oral lesions and conditions associated with human immunodeficiency virus infection in 300 south Indian patients. Oral Dis. 2000; 6:152–157. PMID: 10822358.

21. Ranganathan K, Umadevi M, Saraswathi TR, Kumarasamy N, Solomon S, Johnson N. Oral lesions and conditions associated with human immunodeficiency virus infection in 1000 South Indian patients. Ann Acad Med Singapore. 2004; 33(4 Suppl):37–42. PMID: 15389305.
22. Bravo IM, Correnti M, Escalona L, Perrone M, Brito A, Tovar V, et al. Prevalence of oral lesions in HIV patients related to CD4 cell count and viral load in a Venezuelan population. Med Oral Patol Oral Cir Bucal. 2006; 11:E33–E39. PMID: 16388291.
23. Adurogbangba MI, Aderinokun GA, Odaibo GN, Olaleye OD, Lawoyin TO. Oro-facial lesions and CD4 counts associated with HIV/AIDS in an adult population in Oyo State, Nigeria. Oral Dis. 2004; 10:319–326. PMID: 15533205.

24. Jonsson N, Zimmerman M, Chidzonga MM, Jonsson K. Oral manifestations in 100 Zimbabwean HIV/AIDS patients referred to a specialist centre. Cent Afr J Med. 1998; 44:31–34. PMID: 9675968.
25. Adedigba MA, Ogunbodede EO, Jeboda SO, Naidoo S. Patterns of oral manifestation of HIV/AIDS among 225 Nigerian patients. Oral Dis. 2008; 14:341–346. PMID: 18410577.

26. Arendorf T, Holmes H. Oral manifestations associated with human immunodeficiency virus (HIV) infection in developing countries--are there differences from developed countries? Oral Dis. 2000; 6:133–135. PMID: 10822355.

27. Naidoo S, Chikte U. Oro-facial manifestations in paediatric HIV: a comparative study of institutionalized and hospital outpatients. Oral Dis. 2004; 10:13–18. PMID: 14996288.

28. Bodhade AS, Ganvir SM, Hazarey VK. Oral manifestations of HIV infection and their correlation with CD4 count. J Oral Sci. 2011; 53:203–211. PMID: 21712625.

29. Greenspan JS. Sentinels and signposts: the epidemiology and significance of the oral manifestations of HIV disease. Oral Dis. 1997; 3(Suppl 1):S13–S17. PMID: 9456650.

30. Sabin CA, Phillips AN. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV-1-infected patients? Curr Opin Infect Dis. 2009; 22:191–197. PMID: 19283914.
31. Gaurav S, Keerthilatha PM, Archna N. Prevalence of Oral Manifestations and Their Association with CD4/CD8 Ratio and HIV Viral Load in South India. Int J Dent. 2011; 2011:964278. PMID: 22046186.

32. Nam JH, Noh KL, Pang EO, Kim DY, Kim JH, Chung JA, et al. AIDS-associated Kaposi's sarcoma on left lower retromolar triangle and parapharyngeal area: a case report. J Korean Assoc Oral Maxillofac Surg. 2009; 35:182–186.
Fig. 1
Relationship between oral lesions and the mean baseline clusters of differentiation 4 (CD4) count.
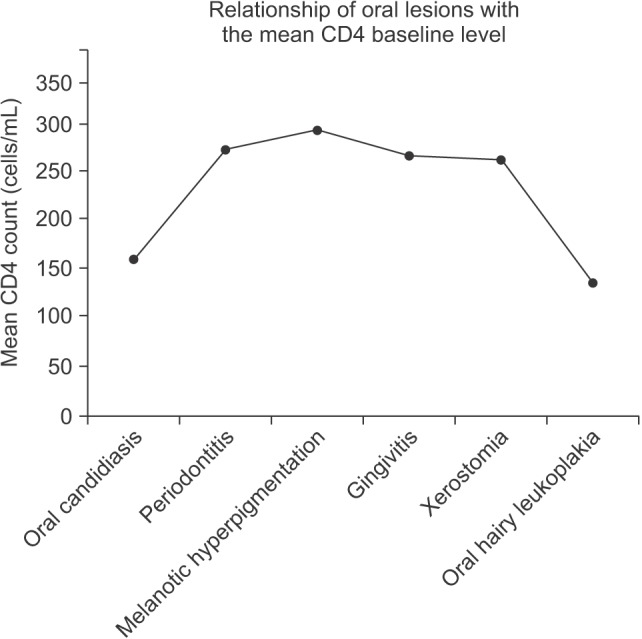
Table 1
Age distribution
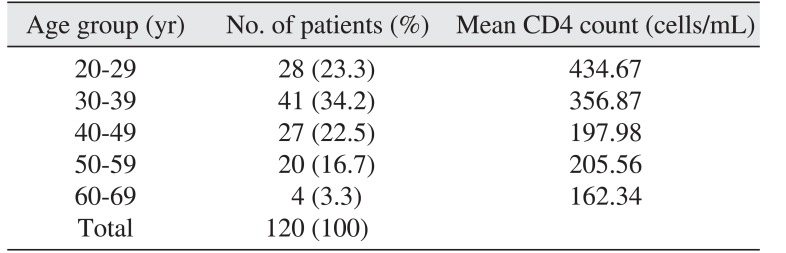
| Age group (yr) | No. of patients (%) | Mean CD4 count (cells/mL) |
|---|---|---|
| 20-29 | 28 (23.3) | 434.67 |
| 30-39 | 41 (34.2) | 356.87 |
| 40-49 | 27 (22.5) | 197.98 |
| 50-59 | 20 (16.7) | 205.56 |
| 60-69 | 4 (3.3) | 162.34 |
| Total | 120 (100) |
Table 2
Oral lesions prevalence in HIV-positive study patients
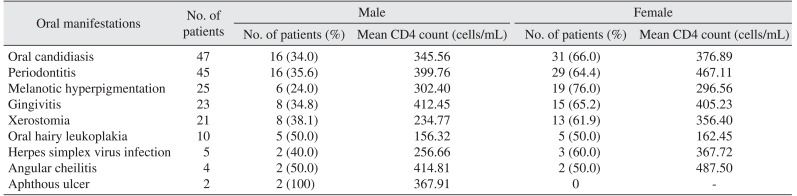
Table 3
Mean baseline CD4 count upon oral lesion presentation




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download