Abstract
Objectives
To evaluate the effectiveness of regenerative tissue matrix (Alloderm) as an oral layer for difficult anterior palatal fistula closure.
Materials and Methods
The authors have tested the feasibility of a novel surgical technique of adding a regenerative tissue matrix (Alloderm) as an oral layer for closure of recalcitrant large anterior palatal fistulae and report the outcome of the first 12 patients in this pilot study. Patients with recurrent large fistula who otherwise would require either a local pedicled flap, free flap, or an obturator were treated with this technique and followed up for at least 6 months to monitor the progress of healing.
Results
Of the 12 patients, 8 patients (66.7%) had complete closure of the fistula, and 2 patients (16.7%) showed reduction in size of the fistula to the extent that symptoms were eliminated, for an overall success rate of 83.3% (10/12 patients). Premature graft loss and recurrence of the fistula were noted in 2 patients (16.7%).
Conclusion
Alloderm provided an adequate barrier allowing healing to occur unimpeded and allowed closure of the palatal fistula. In our experience, this new technique using regenerative tissue matrix as an adjunct to the oral layer in large anterior palatal fistula has an advantage compared to other more invasive complex procedures and has been shown to provide satisfactory results.
Palatal fistula is defined as a communication between the oral and nasal cavities, irrespective of the cause of its occurrence. It is one of the most distressing and challenging complications following cleft palate repair, for both the patient and the surgeon. The incidence of fistula occurrence after palatoplasty has been reported to range from 11% to 34%123. An equally high recurrence rate after fistula closure, ranging from 33% to 50%, was also reported in the literature, which clearly showed the lack of current surgical techniques for fistula repair23456. Palatal fistula forms either due to failure of healing or breakdown following healing. A multitude of factors have been implicated in occurrence and recurrence of palatal fistula including tension along the palate repair, infection, hemorrhage, lack of multilayered closure, and compromised vascularity in the form of either fibrosis or injury to pedicle78. Treatment options vary from no treatment for asymptomatic fistula to non-surgical treatment with obturators to surgical repair. Surgical techniques span the entire reconstructive ladder from local advancement to local pedicled flaps to free tissue transfer depending on type and size of the fistula12679.
Increased recurrence rate is associated with a certain type of fistula, anterior palatal fistula especially in bilateral cleft palate cases24. Multiple previous attempts at repair result in fibrotic palatal tissues that lack adequate blood supply7. This problem can be compounded by lack of adequate tissue surrounding a large fistula7. On average, all patients with large fistula size, anterior palatal location, and history of one or more attempts of fistula repair carry poor prognosis. Palatal obturators, staged procedures such as a tongue flap, or free tissue transfer are the only viable treatment options for such cases.
Regenerative tissue matrix (Alloderm; LifeCell Corporation, Branchburg, NJ, USA) is an acellular dermal matrix derived from donated human skin that undergoes a multistep proprietary process to remove both the epidermis and the cells that can lead to tissue rejection10. This matrix has been used extensively for deep burn patients, for intraoral reconstruction of mucosal defects, and more recently was employed for breast and abdominal wall reconstruction1112. Alloderm also has been used during primary cleft palate repair, when the cleft width exceeded 15 mm, with good results13. It is also employed in palatal fistula repair as an additional layer sandwiched between the mucosal and oral layers in order to hinder recurrence14.
We propose an alternative option of Alloderm in such large-sized and recalcitrant palatal fistulae cases wherein it is used as an inlay graft to provide an oral lining for large fistulae. In our experience, it is usually possible to achieve nasal layer closure in most fistulae using turnover and/or vomer flaps. However, in larger fistulae with lack of adequate surrounding tissues, it might not be possible to achieve a sound oral layer closure. The authors describe and present the preliminary results of using Alloderm as an oral layer on a watertight nasal layer closure in the treatment of large recurrent anterior palatal fistulae.
This clinical study was conducted at Dr. Jeyasekharan Center for Cleft Care, Nagercoil, Tamil Nadu, India from January 2011 to October 2012, and was approved by the Dr. Jeyasekharan Medical Trust Ethical Committee, Tamil Nadu, India. After providing informed written consent, a total of 12 patients with large symptomatic anterior palatal fistula with history of unsuccessful attempt at closure using local palatal flaps were included irrespective of age, sex, and initial classification (i.e., unilateral or bilateral). Simple palatal fistulae that could be easily closed using local palatal flaps or a revision palatoplasty were excluded. All patients underwent operation by a single senior surgeon following a standard surgical protocol.
All patients were treated under general anesthesia, and the surgical site was infiltrated with 2% lignocaine with 1:100,000 adrenaline after placement of a Dingman mouth gag. Incisions were made along the fistulous margins, and the medial and lateral nasal mucosae were elevated from the involved palatal shelf and septum. The turn down flaps were closed with sutures. In all patients, a good watertight nasal layer closure was achieved. Then full thickness mucoperiosteal flaps were raised on both sides based on the availability of tissues. If there was insufficient palatal mucoperiosteum on one side, then minimal undermining of the tissue was performed for the purpose of tucking the graft for stabilization. The rehydrated Alloderm graft was then shaped according to the fistula and placed over the nasal layer with its dermal side facing the nasal mucosa. The palatal mucoperiosteal flaps then were rotated over the graft to cover as much graft as possible without causing any undue pressure. The graft was stabilized by suturing it with the palatal mucoperiosteum with interrupted polyglactin 910 resorbable sutures.(Fig. 1) Intravenous antibiotics were given for 5 days postoperatively. Patients were placed on a semi-solid oral diet for 15 days postoperatively, and proper oral hygiene was maintained without any undue vigorous activity. The patients were followed up regularly for at least 6 months and were assessed with respect to infection, dehiscence, signs of rejection, and fistula recurrence. The schematic representation of the technique of anterior palatal fistula closure and placement of Alloderm is shown in Fig. 2.
A total of 12 patients with symptomatic large anterior fistulae were included in the study. The age ranged from 2 year 3 months to 29 years, with a mean age of 18.58 years.(Table 1) Ten of 12 patients (83.3%) had bilateral cleft deformity, whereas two (16.7%) had unilateral cleft deformity. Nine patients (75.0%) had one previous failed attempt at fistula repair, whereas 3 patients (25.0%) had two previous failed attempts. All patients had persistent symptoms like nasal regurgitation of fluids, fetor oris, recurrent infection, or hypernasal speech associated with the palatal fistula.
Complete closure of palatal fistula was observed in 8 of 12 patients (66.7%), and reduction in the size of the fistula to the extent that symptoms abated was observed in another 2 patients (16.7%).(Fig. 3, 4, 5) In the remaining 2 patients (16.7%), the graft material was totally lost with recurrence of palatal fistula. Both of these failures were in the youngest patients. We speculate that non-compliance with the proper postoperative care may have contributed to failure in these 2 patients.
Overall, the success rate was 83.3% (10 of 12 patients). The Alloderm remained in situ for about 3 to 4 weeks in all patients, after which part of the graft sloughed off. Although total integration of the graft was not seen in any of the case, the results achieved after 6 months are encouraging. The authors observed that Alloderm provided a firm barrier allowing healing to progress unimpeded, thus aiding in closure of large palatal fistula.
Even under the best circumstances, a fistula can develop in the hard palate following cleft palate repair2. Palatal fistula represents a failure of surgical repair as it provides a persistent communication between the oral and nasal cavities, allowing for air and fluid escape. This leads to functional problems like nasal regurgitation of fluids and food particles, malodor, nasal twang, etc. and requires surgical repair for correction78.
A very high failure rate has been associated with attempted palatal fistula repair, sometimes as high as 65%15. This failure rate seems to increase with increasing attempts at repair15. Poor blood supply, limited availability of tissue, scarred adjacent mucosa, and early wound contraction often lead to recurrence of a fistula7. A fistula repair operation should ideally reestablish the normal anatomy with a partition between the nasal and oral cavities lined by epithelium on both sides. Often, closure with one well-vascularized layer provides better results than that with two poorly vascularized layers, which was also shown by Cohen et al.2 in a multivariate analysis of cleft palate fistula. Their study observed that multilayered closure of the palatal fistula had a higher incidence of recurrence (50%) than one-layer closure (25%), but they stated statistical limitations in interpreting the meaning of these numbers. Although inadequate to scientifically establish this fact, these data demonstrate that, by following the basic surgical principles, successful results can be achieved for closure of palatal fistula. Our results also dispel the common belief that two-layered closure with or without interposition material is necessary for successful closure of large palatal fistula.
The present findings support those of Jeffery et al.7, who presented a technique of closure of palatal fistula using a free conchal cartilage graft. They separated nasal and oral layers with an incision and minimum 2 to 3 mm dissection and placed harvested autogenous conchal cartilage in the pocket created with no formal attempt at closure of either the nasal mucosa or palatal mucosa. They achieved complete closure in 79% (11 of 14 cases) of cases and partial closure in the remaining cases. The cartilage graft provided a solid barrier between the healing epithelia, hence avoiding re-establishment of the fistula.
Successful treatment of large anterior palatal fistula has been shown with the use of local pedicled flaps, free tissue transfer, and palatal obturator prosthesis69. Tongue flap is perhaps the most widely used and successful alternative to the problem; however, like free tissue transfer, this procedure had drawbacks of donor-site morbidity, increased surgical time, increased reliance on patient compliance in the postoperative period, increased discomfort to patients, and need for a second surgery for division and inset. Regenerative tissue matrix seems to be a viable alternative to the problem as it has already been described during primary palatoplasty and during fistula closure with good results1314.
Regenerative tissue matrix (Alloderm) is a decellularized dermal matrix derived from human cadaveric skin after undergoing a proprietary procedure to remove epidermis and all the cellular components of the dermis10. This matrix provides a scaffold for tissue in growth, revascularization, and mucosal epithelialization without any evidence of immunologic rejection or donor site morbidity13. It is not a newly developed material and has been successfully, extensively used in other surgical procedures. It was developed for treatment of deep burns cases but is now widely used for breast augmentation, breast reconstruction, abdominal wall reconstruction, hernia repair, pelvic floor reconstruction, etc.1112. In craniofacial areas, it has been used as a soft tissue augmentation material for nasal dorsum and lips and has also been used for resurfacing of intraoral mucosal defects1617. Alloderm has also been successfully used for primary cleft palate repair, when the cleft width exceeded 15 mm13. A previous retrospective study assessed the fistula rate following primary Furlow's palatoplasty using decellularized dermis in which they suggested that a collagen-like barrier can be used to improve the recurrence rate of fistula18.
Kirschner et al.19 used Alloderm as an interpositional material between nasal and oral layers during fistula closure, wherein the oral layer was well closed, and there existed a defect in the nasal lining with eventual closure of all fistulae. Steele and Seagle20 also used Alloderm in fistula repair as an interposition material between oral and nasal layers, and this was compared with the conventional two-layered repair of fistula. Results showed no failure in the Alloderm group compared to the 16.7% failure rate in the control group. They concluded that Alloderm is safe, easy to use, strong, resistant to infection and rejection, and avoids donor-site and associated morbidity. The results are in accordance with our study. In addition, we have used Alloderm as an inlay graft in fistulae wherein a good oral closure was not possible. Although we did not achieve 100% success, our results are encouraging.
In our series, we achieved complete closure of fistula in 8 patients and reduction in fistula size to the extent that symptoms were eliminated in another 2 patients, which can be considered as a successful outcome. This reduction in size to an asymptomatic level has been accepted as a successful outcome by many other authors, and small-sized fistula with no or insignificant symptoms does not justify reoperation245. We did encounter two unsuccessful repairs in children, where the graft was totally lost prematurely with no reduction in size of the fistula. An abnormal location with compromised vascularity, patient non-compliance, and improper postoperative oral hygiene probably led to these graft failures.
We believe that, in successful cases, the graft provided a solid barrier and a membrane effect from saliva and food particles that could otherwise impede the healing process and cause surgical failure and allowed granulation to occur at the defect. In our experience, this new technique of using Alloderm as an adjunct to oral layer in large anterior palatal fistula does have an added advantage compared to other more invasive complex procedures and has been shown to provide satisfactory results. This technique is simple, safe, reasonably successful, and offers a good treatment choice for patients with large anterior palatal fistula. A study employing a larger sample size is currently been performed to substantiate our results.
References
1. Amaratunga NA. Occurrence of oronasal fistulas in operated cleft palate patients. J Oral Maxillofac Surg. 1988; 46:834–838. PMID: 3171742.

2. Cohen SR, Kalinowski J, LaRossa D, Randall P. Cleft palate fistulas: a multivariate statistical analysis of prevalence, etiology, and surgical management. Plast Reconstr Surg. 1991; 87:1041–1047. PMID: 2034725.
3. Muzaffar AR, Byrd HS, Rohrich RJ, Johns DF, LeBlanc D, Beran SJ, et al. Incidence of cleft palate fistula: an institutional experience with two-stage palatal repair. Plast Reconstr Surg. 2001; 108:1515–1518. PMID: 11711920.

4. Abyholm FE, Borchgrevink HH, Eskeland G. Palatal fistulae following cleft palate surgery. Scand J Plast Reconstr Surg. 1979; 13:295–300. PMID: 545672.
5. Rintala AE. Surgical closure of palatal fistulae: follow-up of 84 personally treated cases. Scand J Plast Reconstr Surg. 1980; 14:235–238. PMID: 7209409.
6. Denny AD, Amm CA. Surgical technique for the correction of postpalatoplasty fistulae of the hard palate. Plast Reconstr Surg. 2005; 115:383–387. PMID: 15692340.

7. Jeffery SL, Boorman JG, Dive DC. Use of cartilage grafts for closure of cleft palate fistulae. Br J Plast Surg. 2000; 53:551–554. PMID: 11000068.

8. Wilhelmi BJ, Appelt EA, Hill L, Blackwell SJ. Palatal fistulas: rare with the two-flap palatoplasty repair. Plast Reconstr Surg. 2001; 107:315–318. PMID: 11214043.

9. Schwabegger AH, Hubli E, Rieger M, Gassner R, Schmidt A, Ninkovic M. Role of free-tissue transfer in the treatment of recalcitrant palatal fistulae among patients with cleft palates. Plast Reconstr Surg. 2004; 113:1131–1139. PMID: 15083012.

10. LifeCell website [Internet]. Bridgewater (NJ): LifeCell Corporation;cited 2011 Aug 10. Available from: http://www.lifecell.com.
11. Butler CE, Prieto VG. Reduction of adhesions with composite AlloDerm/polypropylene mesh implants for abdominal wall reconstruction. Plast Reconstr Surg. 2004; 114:464–473. PMID: 15277815.

12. Baxter RA. Intracapsular allogenic dermal grafts for breast implant-related problems. Plast Reconstr Surg. 2003; 112:1692–1696. PMID: 14578804.

13. Clark JM, Saffold SH, Israel JM. Decellularized dermal grafting in cleft palate repair. Arch Facial Plast Surg. 2003; 5:40–44. PMID: 12533137.

14. Cole P, Horn TW, Thaller S. The use of decellularized dermal grafting (AlloDerm) in persistent oro-nasal fistulas after tertiary cleft palate repair. J Craniofac Surg. 2006; 17:636–641. PMID: 16877906.

15. Schultz RC. Management and timing of cleft palate fistula repair. Plast Reconstr Surg. 1986; 78:739–747. PMID: 3538077.

16. Sclafani AP, Romo T 3rd, Jacono AA, McCormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg. 2000; 2:130–136. PMID: 10925439.
17. Rhee PH, Friedman CD, Ridge JA, Kusiak J. The use of processed allograft dermal matrix for intraoral resurfacing: an alternative to split-thickness skin grafts. Arch Otolaryngol Head Neck Surg. 1998; 124:1201–1204. PMID: 9821920.
18. Helling ER, Dev VR, Garza J, Barone C, Nelluri P, Wang PT. Low fistula rate in palatal clefts closed with the Furlow technique using decellularized dermis. Plast Reconstr Surg. 2006; 117:2361–2365. PMID: 16772942.

19. Kirschner RE, Cabiling DS, Slemp AE, Siddiqi F, LaRossa DD, Losee JE. Repair of oronasal fistulae with acellular dermal matrices. Plast Reconstr Surg. 2006; 118:1431–1440. PMID: 17051115.

20. Steele MH, Seagle MB. Palatal fistula repair using acellular dermal matrix: the University of Florida experience. Ann Plast Surg. 2006; 56:50–53. PMID: 16374096.
Fig. 1
Intraoperative photograph shows the placement of the Alloderm (LifeCell Corporation) graft as the oral layer.
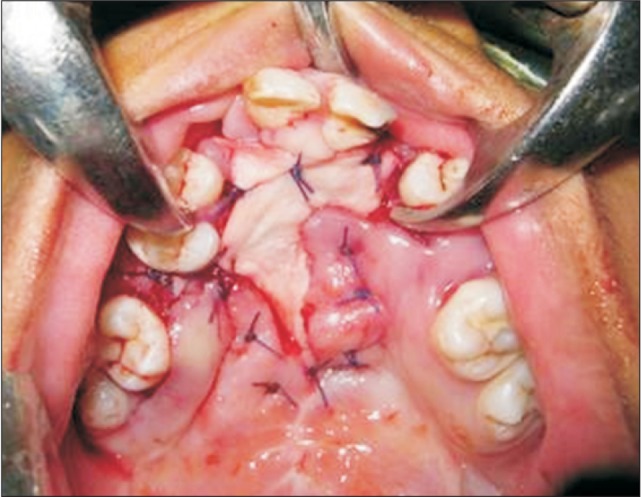
Fig. 2
Schematic representation of the technique used for anterior palatal fistula closure and placement of Alloderm (LifeCell Corporation) as an oral layer. A. Preoperative illustration shows the anterior palatal fistula. B. Planning of turn down flaps around the fistula. C. Closure of the nasal layer in a watertight manner. D. Placement of Alloderm as an oral layer sutured to the palatal mucoperisoteum.
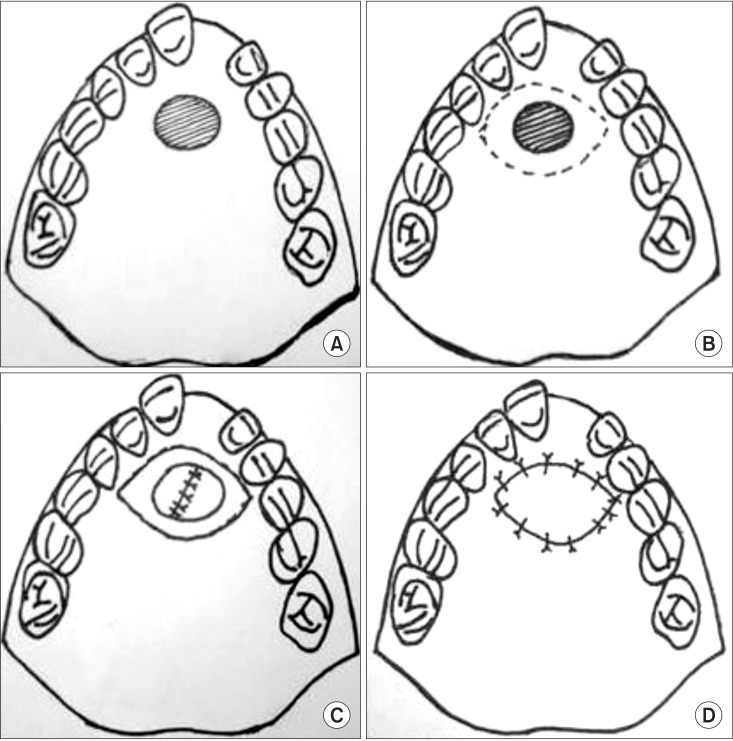
Fig. 3
Clinical photographs of anterior palatal fistula closure in patient 1. A. Preoperative. B. Intraoperative with Alloderm (LifeCell Corporation) in situ. C. Postoperative 6 months with complete closure of the fistula.
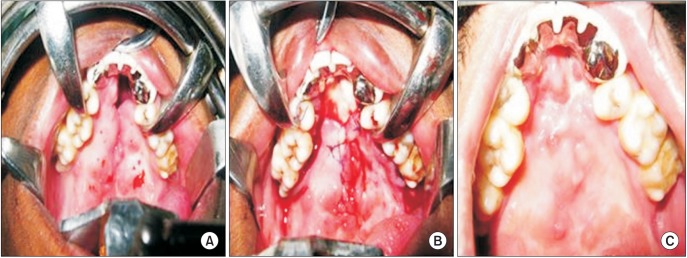
Fig. 4
Clinical photographs of anterior palatal fistula closure in patient 2. A. Preoperative. B. Intraoperative with Alloderm (LifeCell Corporation) in situ. C. Postoperative 6 months with complete closure of the fistula.
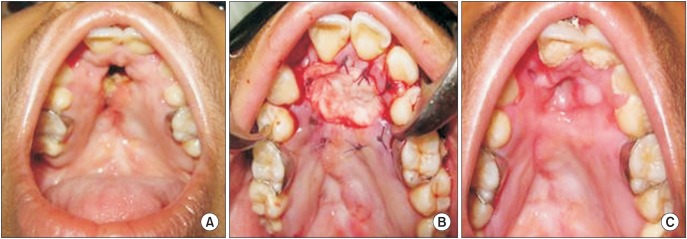
Fig. 5
Clinical photographs of anterior palatal fistula closure in patient 3. A. Preoperative. B. Intraoperative with Alloderm (LifeCell Corporation) in situ. C. Postoperative 6 months with complete closure of the fistula.
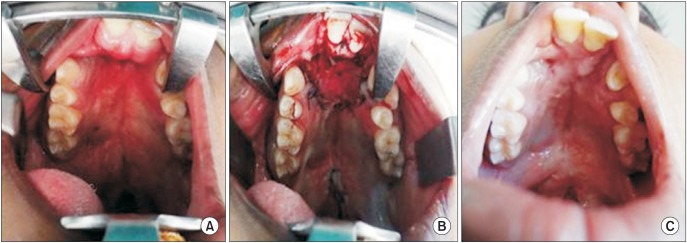
Table 1
Patient data depicting demographic data and outcome of the study population




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download