Abstract
Objectives
This article describes our experience with neck dissection in 10 patients with oral squamous cell carcinoma.
Materials and Methods
Between January 2007 and October 2009, 10 patients underwent primary surgery for the treatment of squamous cell carcinoma of the oral cavity. For patients with N0 disease on clinical exam, selective neck dissection (SND [I-III]) was performed. In patients with palpable cervical metastases (N+), modified radical neck dissections were performed, except in one patient in whom SND (I-III) was performed. The histopathologic reports were reviewed to assess the surgical margins, the presence of extra-capsular spread, perineural invasion, and lymphatic invasion.
Results
On histopathologic examination, positive soft tissue margins were found in three patients, and regional lymph node metastases were present in five of the ten patients. Perineural invasion was noted in five patients, and extra nodal spread was found in four patients. Regional recurrence was seen in two patients and loco-regional recurrence plus distant metastasis to the tibia was observed in one patient. During the study period, three patients died. Seven patients remain free of disease to date.
Conclusion
Histopathological evaluation provides important and reliable information for disease staging, treatment planning, and prognosis. The philosophy of neck dissection is evolving rapidly with regard to the selectivity with which at-risk lymph node groups are removed. The sample size in the present study is small, thus, caution should be employed when interpreting these results.
The treatment of oral squamous cell carcinoma is directed at controlling the primary tumor and regional neck metastases, and neck dissection is an integral treatment component12. Lymph node metastasis is found in more than 50% of patients with oral squamous cell carcinoma3. Notably, the status of cervical metastasis is the single most important prognostic factor in survival of patients with oral squamous cell carcinoma456, with the cure rates dropping to nearly half with regional lymph node involvement678.
The decision to perform neck dissections is based upon the knowledge of nodal metastasis patterns and risk factors for neck metastasis such as tumor site, size, and thickness6. In patients clinically staged as N0, the lymph node levels at risk are I-III, while in N+ patients, the levels at risk are I-IV69. Notably, the posterior triangle (level V) is rarely involved by metastases from oral carcinomas6910. This information is helpful in formulating a rational approach to surgical management of the neck6. In clinically N0 patients, selective neck dissection (SND [I-III]) is adequate. SND (I-III) is therapeutic for pathologically negative nodes and also provides necessary information for whether postoperative radiation therapy is required for pathologically positive nodes61112. In N+ patients, modified radical neck dissection (MRND) with preservation of the spinal accessory nerve, if possible, is advocated612.
The concept of neck dissection in head and neck carcinoma was first introduced by Crile13 in 1906. Since then, various techniques to preserve the lymph nodes, the spinal accessory nerve, the internal jugular vein, and the sternocleidomastoid muscle have been developed. Most recently, SND has been accepted as the most successful staging and therapeutic procedure for oral squamous cell carcinoma in the clinically negative neck14. SND is also being performed in patients with N+ necks and limited metastasis confined to the neck15. The philosophy of neck dissection is evolving rapidly with regard to the selectivity of at-risk lymph node group removal. In the future, more SND approaches, such as super SNDs, might replace SND. In this paper, we present our experience with neck dissection in 10 patients with oral squamous cell carcinoma, and we also present a review of the literature.
Between January 2007 and October 2009, 10 patients underwent primary surgery for the treatment of squamous cell carcinoma of the oral cavity. The institutional review board of our institution approved the current study, and the study was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent. Data regarding the age, sex, primary tumor site, and TNM stage were recorded. Preoperative biopsies of the primary tumors were collected to confirm the clinical diagnoses. TNM staging was assigned according to the 2002 American Joint Committee on Cancer classifications. Ultrasonography and computed tomography scan of the neck were performed to determine the extent of neck metastasis. Distant metastases were excluded by further clinical examination of distant nodal sites and by chest radiography. Pre-anesthetic testing and medical evaluations were performed for each patient as needed.
Surgical dissection of the cervical lymph nodes at risk of metastasis was undertaken as part of the management of the primary tumor. For patients with clinically N0 necks, SND (I-III) was performed. In patients with palpable cervical metastases (N+), MRND was performed, except in one patient in whom SND (I-III) was performed. SND (I-III) was completed in six patients, and MRND was completed in four patients. The histopathologic reports were assessed for surgical margins, the presence of extracapsular spread, perineural invasion, and lymphatic invasion. Postoperative radiation therapy to the neck was used in patients with involved or nearly involved margins of excision, extranodal tumor spread, multiple positive lymph nodes in the neck, and in those with poorly differentiated pathology with perineural or perivascular spread. Five patients were treated with surgery followed by radiotherapy. In two patients, surgery was followed by radiation therapy and chemotherapy. Three patients were treated by surgery alone, as there was no postoperative indication for radiotherapy. Patients were followed up every four months for the first two years and every six months thereafter.
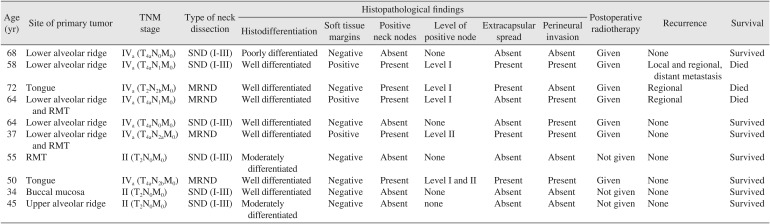
The present series was comprised of six men and four women ranging in age from 34 to 72 years. Patient age, tumor site, TMN stage, level of positive nodes, treatment, histopathological findings, and survival are summarized in Table 1. On histopathologic examination, negative soft tissue margins were found in seven patients, and positive soft tissue margins were found in three patients. Positive bony margins were found in only one patient out of seven in whom bone resections were performed.
Regional lymph node metastases were present in five out of ten patients. Perineural invasion was noted in five patients, and extra nodal spread was noted in four patients. Regional recurrence was noted in two patients, and loco-regional recurrence with distant metastasis to the tibia was observed in one patient. During the study period, three patients died. Of these, one patient died of distant metastasis, one patient died of regional recurrence, and one patient died of uncontrolled hypertension. Seven patients remain free of disease to date.
The successful treatment of oral squamous cell carcinoma hinges on management of the neck because metastasis to the neck is the single most important prognostic factor46716171819. Interestingly, the patterns of nodal spread in the neck are relatively predictable6. Specifically, neck levels I, II, and III are at the greatest risk of nodal metastasis from primary squamous cell carcinoma of the oral cavity9. Tumors of the tongue have the highest incidence of neck metastasis, followed by the floor of the mouth, the lower gums, the buccal mucosa, the upper gums, the hard palate, and the lips620. The incidence of pathologically-proven metastasis in the clinically N0 neck follows a similar pattern. Tumors of the upper gum, hard palate, and lips have such a low rate of occult metastasis that elective treatment of the neck is unnecessary. Additionally, the posterior triangle (level V) is seldom involved by metastases from these lesions610.
Management of the N0 neck in oral squamous cell carcinoma has been debated extensively, but several authors support the use of elective neck dissection21222324. SND (I-III) or supraomohyoid neck dissection consists of removing the nodal regions I, II, and III2526. These approaches remove the nodes at the highest risk of involvement from a primary tumor originating in the oral cavity27. SND (I-III) is the standard staging procedure and may even be considered therapeutic for patients with N0 oral and oropharyngeal cancers28. It is widely accepted as the appropriate SND approach for patients with oral cavity cancer and a clinically negative neck112529. In our series, SND (I-III) was performed in five patients with clinically negative necks. After five years of follow-up, no recurrence has been noted to date.
Cunningham et al.30 supported the use of elective neck dissection in patients with stage I and II oral cavity carcinoma. Furthermore, Kligerman et al.21 conducted a study to determine the indication for elective neck dissection in patients with early oral cavity squamous cell carcinoma. Sixty-seven patients were stratified by stage (T1 and T2N0), and patients in each stage were randomized to receive resection alone or resection plus elective supraomohyoid neck dissection. The disease-free survival rates at 3.5 years for resection alone and resection plus elective supraomohyoid neck dissection were 49% and 72%, respectively. They concluded that neck dissection was mandatory in early-stage oral squamous cell carcinoma due to the superior survival rates compared to resection alone and the poor salvage rate. In considering the results from the above authors, we performed elective neck dissections in patients with early-stage squamous cell carcinoma.
Tumors arising from the tongue and floor of the mouth have a high propensity for early metastasis, regardless of their size and differentiation. Unless the treatment of choice for the primary lesion is radiotherapy, elective neck dissection with removal of lymph node levels I through III (and level IV for tongue cancer) is the minimum recommended treatment for N0 squamous cell carcinoma of the oral cavity26. Of note, Byers et al.31 observed skip metastases to level IV in squamous cell carcinoma of the tongue at a rate of 15% and suggested that level IV should be included in SND. In addition, Woolgar32 also advocated for this approach. However, Khafif et al.33 found that the rate of metastasis to the undissected level IV was only 2% in tongue carcinoma, and they concluded that metastasis to level IV lymph nodes was rare in patients with T1-T3 or N0 oral tongue cancer. Thus, they proposed that dissection of these nodes should be performed hisonly when there is intraoperative suspicion of metastasis in levels II or III.
SND (I-III) has been used extensively as a staging procedure in patients with N0 oral squamous cell carcinoma, and it is also a potentially curative procedure in selected patients with limited metastatic disease of the neck11. Pathological studies of lymph node metastasis suggest that the use of SND is also appropriate in some patients with clinically obvious cervical lymph node metastases. When indicated, the application of postoperative radiation therapy further reduces the rate of regional failure in patients following SND15. Furthermore, Kolli et al.34 assessed the potential role of SND (I-III) in patients with clinically positive nodes. The overall regional control rates achieved with SND (I-III) in patients with pathologically negative vs positive nodes in the neck were 88% vs 71%, respectively. These results indicated that SND (I-III) alone was inadequate treatment for patients with pathologically confirmed, clinically positive nodes. Adjuvant radiation therapy in these patients improved regional control, however. Additionally, Andersen et al.35 conducted a study to determine the oncologic efficacy of SND in patients with nodepositive squamous cell carcinoma of the head and neck. They concluded that SND can be performed safely and effectively in appropriately chosen patients with clinically node positive metastasis in the neck from squamous cell carcinoma of the upper aerodigestive tract. Regional control rates comparable to those of more comprehensive operations can be achieved in appropriately selected cases. For example, Traynor et al.36 suggested that the use of SND could be extended to N2B and N2C disease in the absence of massive lymphadenopathy, nodal fixation, gross extracapsular spread, or a history of previous neck surgery. However, Spiro et al.25 critically assessed supraomohyoid neck dissection and SND (I-III) and stated that when nodal disease was clinically obvious, treatment failure was more frequent, even with the addition of postoperative radiotherapy. In the present series, SND (I-III) was performed in one patient with clinically obvious nodes, and even with the addition of postoperative radiotherapy, the treatment failed.
Nowadays, attempts are being made to replace MRND with SND for early node positivity37. It is possible that in the future, SND combined with adjuncts such as radiotherapy or chemotherapy will become the standard of care for advanced nodal disease20. In N0 and N+ disease with metastasis identified at a single anterior level, the prevalence of level V metastasis remains low. Therefore, SND is adequate management in these cases. The low likelihood of metastasis to level V, even in N+ disease, should be considered when performing neck dissection for oral squamous cell carcinoma10.
The neck should always be treated in patients who have larger T3 and T4 cancers because of the high incidence of nodal metastasis1. If there is palpable nodal disease (N+), removal of levels IV and V is also necessary. However, exceptions may be considered for nodal disease confined to levels I or II by gross examination intraoperatively. Under these circumstances, selective removal of levels I through IV is more appropriate26. In our series, MRND was performed in four patients with N+ necks.
The histologic evaluation of head and neck cancers provides important and reliable information for disease staging, treatment planning, and prognosis38. In their study on the prognostic value of lymph node involvement in oral cancers, Tankéré et al.39 found that lymph node involvement was present in 52.6% of patients. In our patients, lymph node metastasis was present in 5 out of 10 patients. Rahima et al.40 found that metastatic lymph nodes were confirmed histologically in 80.6% of patients who underwent therapeutic neck dissection. However, the clinical assessment of lymph nodes has pitfalls. Specifically, the incidence of carcinoma in clinically negative nodes varies from 15% to 38%38. Woolgar32 explored the pathology of the N0 neck and found metastasis in 21% of patients with N0 necks and intraoral/oropharyngeal carcinoma.
The factors responsible for treatment failure at the primary site and in the neck, as studied by Shah et al.41 are female gender, higher stage, tumors with deep invasion, positive surgical margins, multiple involved lymph nodes at multiple levels, extracapsular extension of disease in the cervical lymph nodes, and involvement of soft tissues in the neck. Local radicality and whether the surgical margins are positive or clean are prognostically significant in the surgical management of oral cancer. Reports differ concerning the occurrence of positive margins, with rates varying between 0% and 52% depending on the location and extension of the primary tumor42. In the present study, positive surgical margins were found in three patients. These patients were managed by postoperative radiotherapy after consultation with a radiotherapist. Surgery was not performed in these cases, as these patients were reluctant to undergo an additional procedure.
Histologic demonstration of extranodal tumor spread in metastatic lymph nodes is thought to be an important prognostic factor for recurrence in squamous cell carcinomas of the head and neck43. Leemans et al.44 studied recurrence of head and neck cancer at the primary site in relation to the histopathologic involvement of the neck. They found that extracapsular spread was present in 45.1% patients. In their study on the impact of extranodal spread of lymph node metastases in patients with oral cancer, Shingaki et al.43 observed that extranodal spread was present in 46% of patients. In the current study, extranodal spread was noted in 4 out of 10 patients. Furthermore, Shingaki et al.43 reported that the rate of neck failure in patients without extranodal spread was 14%, and the rate was 18% in patients with extranodal spread. In the present series, half of the patients with extranodal spread developed neck recurrence.
Rahima et al.40 conducted a study to elucidate the occurrence of perineural invasion in patients with squamous cell carcinoma of the oral cavity and oropharynx, and they examined the effect of perineural invasion on survival, the incidence of local recurrence, regional recurrence, and distant metastasis. They found perineural invasion in 25.7% patients. However, in the current series, perineural invasion was found in five patients. Rahima et al.40 found that only perineural invasion was significantly associated with regional recurrence. In the present study, three patients had regional recurrence, and of these, two also had perineural invasion. Furthermore, these authors also found an association between perineural invasion and distant metastasis40. In the present series, distant metastasis was found in one patient, and perineural invasion was also present in this patient. The relationship between perineural invasion and the incidence of local recurrence has been reported by many investigators40. In the current series, one patient developed local recurrence, and this patient also had perineural invasion.
Arduino et al.45 performed a retrospective study of 334 cases exploring the independent clinical and histopathologic prognostic factors of oral squamous cell carcinoma. They found recurrence in almost 30% of their patients. The recurrence varies from 16% to 42%46. In the present series, three patients had recurrence. One patient had recurrence at the primary site and in the neck, and two patients had regional recurrence. All three patients had higher T stages, and lymph node metastases were present. Of these three patients, two had positive surgical margins. Extracapsular spread was present in two of the three patients, and perineural invasion was present in two patients.
The three most significant factors impacting the survival rate of oral cancer patients are TNM stage, local recurrence after surgery, and metastasis to cervical lymph nodes after surgery46. The overall five-year survival rate of oral squamous cell carcinoma patients as studied by Oh et al.47 was 66.90%. In the present study, seven patients survived, but three patients died of the disease.
In the past decades, significant refinements have been made in the philosophy of neck dissection, emphasizing the need for functional conservation to improve the quality of life of patients with oral squamous cell carcinoma. Nowadays, MRND is being replaced by SND, and trends suggest that the future will offer even more SND approaches, such as super SNDs. Super SND involves the compartmental removal of fibrofatty tissue contents within the defined boundaries of two or fewer contiguous neck levels. It is indicated mainly for elective treatment of the clinically N0 neck48, but can be considered for salvage treatment of persistent lymph node disease after chemoradiotherapy49. We believe that if comparable results can be achieved with a more SND, then the quality of life in patients with oral cancer may continue to improve.
This article describes our experience with neck dissection in ten patients with oral squamous cell carcinoma. Histopathological evaluation provides important and reliable information for disease staging, treatment planning, and prognosis. The philosophy of neck dissection is evolving rapidly with regard to the selectivity with which at-risk lymph node groups are removed. The sample size in the present study is small; hence, it may be prudent to exercise caution when interpreting the results.
References
1. Campana JP, Meyers AD. The surgical management of oral cancer. Otolaryngol Clin North Am. 2006; 39:331–348. PMID: 16580915.

2. Khatri VP, Loree TR. A logical and stepwise operative approach to radical neck dissection. Arch Surg. 2002; 137:345–351. PMID: 11888465.

3. Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital. 2007; 27:113–117. PMID: 17883186.
4. Persky MS, Lagmay VM. Treatment of the clinically negative neck in oral squamous cell carcinoma. Laryngoscope. 1999; 109:1160–1164. PMID: 10401861.

5. Jalisi S. Management of the clinically negative neck in early squamous cell carcinoma of the oral cavity. Otolaryngol Clin North Am. 2005; 38:37–46. PMID: 15649497.

6. Shah JP, Andersen PE. Evolving role of modifications in neck dissection for oral squamous carcinoma. Br J Oral Maxillofac Surg. 1995; 33:3–8. PMID: 7718525.

7. Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990; 160:405–409. PMID: 2221244.

8. Chiesa F. Centenary of Crile's operation. From radical to selective neck dissection. Acta Otorhinolaryngol Ital. 2006; 26:307–308. PMID: 17633148.
9. Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990; 66:109–113. PMID: 2354399.

10. Davidson BJ, Kulkarny V, Delacure MD, Shah JP. Posterior triangle metastases of squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1993; 166:395–398. PMID: 8214300.

11. Spiro RH, Morgan GJ, Strong EW, Shah JP. Supraomohyoid neck dissection. Am J Surg. 1996; 172:650–653. PMID: 8988669.

12. Carlson ER, Cheung A, Smith B, Pfohl C. Neck dissections for oral/head and neck cancer: 1906-2006. J Oral Maxillofac Surg. 2006; 64:4–11. PMID: 16360851.

13. Crile GW. Excision of the cancer of the head and neck: with special reference to the plan of dissection based on one hundred and thirty two operations. JAMA. 1906; 47:1780–1786.
14. Saffold SH, Wax MK, Nguyen A, Caro JE, Andersen PE, Everts EC, et al. Sensory changes associated with selective neck dissection. Arch Otolaryngol Head Neck Surg. 2000; 126:425–428. PMID: 10722022.

15. Ferlito A, Rinaldo A, Robbins KT, Leemans CR, Shah JP, Shaha AR, et al. Changing concepts in the surgical management of the cervical node metastasis. Oral Oncol. 2003; 39:429–435. PMID: 12747966.

16. Andersen PE, Shah JP, Cambronero E, Spiro RH. The role of comprehensive neck dissection with preservation of the spinal accessory nerve in the clinically positive neck. Am J Surg. 1994; 168:499–502. PMID: 7977984.

17. Sivanandan R, Kaplan MJ, Lee KJ, Lebl D, Pinto H, Le QT, et al. Long-term results of 100 consecutive comprehensive neck dissections: implications for selective neck dissections. Arch Otolaryngol Head Neck Surg. 2004; 130:1369–1373. PMID: 15611394.
18. Spiro RH, Strong EW, Shah JP. Classification of neck dissection: variations on a new theme. Am J Surg. 1994; 168:415–418. PMID: 7977963.

19. McGuirt WF Jr, Johnson JT, Myers EN, Rothfield R, Wagner R. Floor of mouth carcinoma. The management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1995; 121:278–282. PMID: 7873143.

20. Chummun S, McLean NR, Ragbir M. Surgical education: neck dissection. Br J Plast Surg. 2004; 57:610–623. PMID: 15380694.

21. Kligerman J, Lima RA, Soares JR, Prado L, Dias FL, Freitas EQ, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994; 168:391–394. PMID: 7977957.

22. Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol. 2011; 47:320–324. PMID: 21459661.

23. Lydiatt DD, Robbins KT, Byers RM, Wolf PF. Treatment of stage I and II oral tongue cancer. Head Neck. 1993; 15:308–312. PMID: 8360052.

24. Andersen PE, Cambronero E, Shaha AR, Shah JP. The extent of neck disease after regional failure during observation of the N0 neck. Am J Surg. 1996; 172:689–691. PMID: 8988679.

25. Spiro JD, Spiro RH, Shah JP, Sessions RB, Strong EW. Critical assessment of supraomohyoid neck dissection. Am J Surg. 1988; 156:286–289. PMID: 3177752.

26. Robbins KT. Classification of neck dissection: current concepts and future considerations. Otolaryngol Clin North Am. 1998; 31:639–655. PMID: 9687326.
27. Byers RM. Modified neck dissection. A study of 967 cases from 1970 to 1980. Am J Surg. 1985; 150:414–421. PMID: 4051103.
28. Carvalho AL, Kowalski LP, Borges JA, Aguiar S Jr, Magrin J. Ipsilateral neck cancer recurrences after elective supraomohyoid neck dissection. Arch Otolaryngol Head Neck Surg. 2000; 126:410–412. PMID: 10722018.

29. O'Brien CJ, Traynor SJ, McNeil E, McMahon JD, Chaplin JM. The use of clinical criteria alone in the management of the clinically negative neck among patients with squamous cell carcinoma of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2000; 126:360–365. PMID: 10722009.
30. Cunningham MJ, Johnson JT, Myers EN, Schramm VL Jr, Thearle PB. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am J Surg. 1986; 152:361–366. PMID: 3766864.

31. Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of "skip metastases" in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997; 19:14–19. PMID: 9030939.

32. Woolgar JA. Pathology of the N0 neck. Br J Oral Maxillofac Surg. 1999; 37:205–209. PMID: 10454028.

33. Khafif A, Lopez-Garza JR, Medina JE. Is dissection of level IV necessary in patients with T1-T3 N0 tongue cancer? Laryngoscope. 2001; 111:1088–1090. PMID: 11404626.

34. Kolli VR, Datta RV, Orner JB, Hicks WL Jr, Loree TR. The role of supraomohyoid neck dissection in patients with positive nodes. Arch Otolaryngol Head Neck Surg. 2000; 126:413–416. PMID: 10722019.

35. Andersen PE, Warren F, Spiro J, Burningham A, Wong R, Wax MK, et al. Results of selective neck dissection in management of the node-positive neck. Arch Otolaryngol Head Neck Surg. 2002; 128:1180–1184. PMID: 12365890.

36. Traynor SJ, Cohen JI, Gray J, Andersen PE, Everts EC. Selective neck dissection and the management of the node-positive neck. Am J Surg. 1996; 172:654–657. PMID: 8988670.

37. Harish K. Neck dissections: radical to conservative. World J Surg Oncol. 2005; 3:21. PMID: 15836786.
38. Gillies EM, Luna MA. Histologic evaluation of neck dissection specimens. Otolaryngol Clin North Am. 1998; 31:759–771. PMID: 9735105.

39. Tankéré F, Camproux A, Barry B, Guedon C, Depondt J, Gehanno P. Prognostic value of lymph node involvement in oral cancers: a study of 137 cases. Laryngoscope. 2000; 110:2061–2065. PMID: 11129021.
40. Rahima B, Shingaki S, Nagata M, Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:423–431. PMID: 15088027.

41. Shah JP, Cendon RA, Farr HW, Strong EW. Carcinoma of the oral cavity: factors affecting treatment failure at the primary site and neck. Am J Surg. 1976; 132:504–507. PMID: 1015542.
42. Kovács AF. Relevance of positive margins in case of adjuvant therapy of oral cancer. Int J Oral Maxillofac Surg. 2004; 33:447–453. PMID: 15183407.

43. Shingaki S, Nomura T, Takada M, Kobayashi T, Suzuki I, Nakajima T. The impact of extranodal spread of lymph node metastases in patients with oral cancer. Int J Oral Maxillofac Surg. 1999; 28:279–284. PMID: 10416895.

44. Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994; 73:187–190. PMID: 8275423.

45. Arduino PG, Carrozzo M, Chiecchio A, Broccoletti R, Tirone F, Borra E, et al. Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: a retrospective study of 334 cases. J Oral Maxillofac Surg. 2008; 66:1570–1579. PMID: 18634942.

46. Geum DH, Roh YC, Yoon SY, Kim HG, Lee JH, Song JM, et al. The impact factors on 5-year survival rate in patients operated with oral cancer. J Korean Assoc Oral Maxillofac Surg. 2013; 39:207–216. PMID: 24471047.

47. Oh MS, Kang SH, Kim HJ, Zhenglin Z, Ryu JI, Nam W, et al. Overall five-year survival rate in squamous cell carcinoma of oral cavity. J Korean Assoc Oral Maxillofac Surg. 2009; 35:83–88.
48. Suárez C, Rodrigo JP, Robbins KT, Paleri V, Silver CE, Rinaldo A, et al. Superselective neck dissection: rationale, indications, and results. Eur Arch Otorhinolaryngol. 2013; 270:2815–2821. PMID: 23321797.

49. Robbins KT, Shannon K, Vieira F. Superselective neck dissection after chemoradiation: feasibility based on clinical and pathologic comparisons. Arch Otolaryngol Head Neck Surg. 2007; 133:486–489. PMID: 17520763.
Table 1
Details of cases presented




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download