Abstract
Figures and Tables
Fig. 1
CD1a-positive Langerhans cells, CD8-positive T lymphocytes, and CD68-positive macrophages in the oral floor mucosa. Immunohistochemistry of CD8 (A, D), CD68 (B, E), and CD1a (C, F). Panel (A-C) display the same mucosal fold using adjacent sections at the same magnification (scale bar in panel A=1 mm), while panels (D-F) correspond to square in panels (A-C), respectively at the same magnification (scale bar in panel D=0.1 mm). Thus, the positive cells do not show maximum density in panels (E) and (F). Some of suppressor lymphocytes have migrated into the epithelium (D), in contrast to macrophages (E). The epithelium contains abundant spherical Langerhans cells (arrows in panel F).

Fig. 2
CD1a-positive Langerhans cells, CD8-positive T lymphocytes and CD68-positive macrophages in the lower lip. Immunohistochemistry of CD8 (A, E), CD68 (B, F), and CD1a (C, D). Panels (A-C) display the same site using adjacent sections at the same magnification (scale bar in panel A=1 mm), while panels (D-F) correspond to square in panels (A-C), respectively at the same magnification (scale bar in panel D=0.1 mm). Thus, the positive cells do not show maximum density (E, F).

Fig. 3
CD1a-positive Langerhans cells, CD8-positive T lymphocytes, and CD68-positive macrophages in the palpebral conjunctiva. Immunohistochemistry of CD8 (A, D), CD68 (B, E), and CD1a (C, F). Panel (A-C) display the same mucosal grooves using adjacent sections at the same magnification (scale bar in panel A=1 mm), while panels (D-F) correspond to square in panels (A-C), respectively at the same magnification (scale bar in panel D=0.1 mm). Thus, the positive cells do not have maximum density (D, E). A few suppressor lymphocytes and macrophages have migrated into the epithelium (arrows in panels D and E). The epithelium contains abundant pyramidal or dendritic Langerhans cells (F).
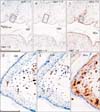
Fig. 4
CD1a-positive Langerhans cells, CD8-positive T lymphocytes, and CD68-positive macrophages in the anal canal. Immunohistochemistry of CD8 (A, D, E), CD68 (B, F, G), and CD1a (C, H). Panel (A-C) display the same mucosal groove using near sections at the same magnification (scale bar in panel A=1 mm), while panels (D), (F), and (H) correspond to square in panels (A-C) at the same magnification (scale bar in panel D=0.1 mm). Thus, the positive cells do not show maximum density (D, F). Panels (E) and (G) (scale bar in panel E=0.1 mm) show submucosal tissue around a vein (corresponding to circle in panels A and B, respectively). Suppressor lymphocytes as well as macrophages are concentrated not in and along the epithelium, but near the submucosal vessels (D-G). Langerhans cells extend in parallel with (not toward) the epithelial surface (H).

Fig. 5
CD1a-positive Langerhans cells, CD8-positive T lymphocytes and CD68-positive macrophages in the penile skin. Immunohistochemistry of CD8 (A, D), CD68 (B, E), and CD1a (C, F). Panel (A-C) display the bottom of the coronary sulcus or groove under the penile prepuce using adjacent sections at the same magnification (scale bar in panel A=1 mm), while panels (D-F) correspond to square in panels (A-C), respectively at the same magnification (scale bar in panel D=0.1 mm). Thus, the positive cells do not show maximum density (E, F). Abundant suppressor lymphocytes have migrated into the epithelium (D), in contrast to macrophages (E). The epithelium contains pyramidal Langerhans cells (F).

Table 1
Numbers of CD1a-positive Langerhans cells in a field of ×20 objective

The counting was performed at the hot spot (a site with highest density) in the section. Ages of each of the cadavers when he died were 85 (specimen A), 85 (B), 86 (C), 88 (D), 89 (E), 91 (F), 94 (G) and 95 (H), respectively. ND, a failure in the immunostaining or the other histological procedure. a)Fig. 3. b)Fig. 2. c)Fig. 5. d)Fig. 4. e)Fig. 1.
Table 2
Numbers of CD8-positive lymphocytes in a field of ×20 objective

The same specimens as shown in Table 1. The counting was performed at the hot spot (a site with the highest density) in the section. Greater number (smaller number) for the oral mucosa, conjunctiva and penis: the greater number indicates total cell number per mm in the epithelium as well as in the submucosal or subcutaneous tissue within 0.1 mm from the epithelial base. The smaller number indicates cell numbers migrating into the epithelium. In the anus, CD8-positive lymphocytes were not seen in the epithelium. ND, a failure in the immunostainig or the other histological procedure. a)Fig. 3. b)Fig. 2. c)Fig. 5. d)Fig. 4. e)Fig. 1.
Table 3
Numbers of CD68-positive macrophages in a field of ×20 objective

The same specimens as shown in Table 1. The counting was performed at the hot spot (a site with the highest density) in the section. Numbers with parenthesis for the oral floor (specimens B, D, and E), the lip (specimen D and E) and conjunctiva (all specimens): cell numbers migrating into the epithelium. In the anus and penis, CD68-positive macrophages were not seen in the epithelium. ND, a failure in the immunostaining or the other histological procedure. a)Fig. 3. b)Fig. 2. c)Fig. 5. d)Fig. 4. e)Fig. 1.
Table 4
Comparison of numbers of CD1a-positive Langerhans cells

| Oral floor | Lip | Conjunctiva | Anus | Penis | |
|---|---|---|---|---|---|
| Oral floor | - | - | - | - | - |
| Lip | - | - | - | - | - |
| Conjunctiva | - | - | - | - | - |
| Anus | - | - | - | - | - |
| Penis | - | - | - | - | - |
Table 5
Comparison of numbers of CD8-positive lymphocytes

| Oral floor | Lip | Conjunctiva | Anus | Penis | |
|---|---|---|---|---|---|
| Oral floor | - | * | - | * | - |
| Lip | * | - | - | - | * |
| Conjunctiva | - | - | - | * | - |
| Anus | * | - | * | - | * |
| Penis | - | * | - | * | - |
Table 6
Comparison of numbers of CD68-posirive macrophages

| Oral floor | Lip | Conjunctiva | Anus | Penis | |
|---|---|---|---|---|---|
| Oral floor | - | - | - | * | * |
| Lip | - | - | - | * | * |
| Conjunctiva | - | - | - | * | * |
| Anus | * | * | * | - | - |
| Penis | * | * | * | - | - |
Table 7
Summary of the distribution of CD1a-positive Langerhans cells, CD8-positive T lymphocytes, and CD68-positive macrophages

Langerhans cell, CD1a-postivie cell; lymphocyte, CD8-positive cell; macrophage, CD68-posiive cell. Evenly or concentrated: they were evenly distributed along the epithelium or concentrated at the bottom of the skin groove (penile skin) or in finger-like protrusions of the stratum spinosum between papillae (anal canal). a)The dendritic cells were not directing toward the epithelial surface but lay in parallel with the epithelial surface.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download