Abstract
Asthma is characterized by chronic inflammation, goblet cell hyperplasia, the aberrant production of the Th2 cytokines, and eosinophil infiltration into the lungs. In this study, we examined the effects of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on ovalbumin (OVA)-induced asthma by evaluating Th1/Th2 cytokine levels, histopathologic analysis, and compound 48/80-induced systemic anaphylaxis and mast cell activation, focusing on the histamine release from rat peritoneal mast cells. Baicalein, wogonin, and S. baicalensis ethanol extract also decreased the number of inflammatory cells especially eosinophils and downregulated peribronchial and perivascular inflammation in the lungs of mice challenged by OVA. Baicalein, wogonin, and S. baicalensis ethanol extract significantly reduced the levels of tumor necrosis factor α, interleukin (IL)-1β, IL-4, IL-5 and the production of OVA-specific IgE and IgG1, and upregulated the level of interferon-γ and OVA-specific IgG2a. In addition, oral administration of baicalein, wogonin, and S. baicalensis ethanol extract inhibited compound 48/80-induced systemic anaphylaxis and plasma histamine release in mice. Moreover, baicalein, wogonin, and S. baicalensis ethanol extract suppressed compound 48/80-induced mast cell degranulation and histamine release from rat peritoneal mast cells. Conclusively, baicalein and wogonin as major flavonoids of S. baicalensis may have therapeutic potential for allergic asthma through modulation of Th1/Th2 cytokine imbalance and histamine release from mast cells.
Bronchial asthma is an increasingly prevalent and often severe disease characterized by variable airway obstruction in response to allergen, airway eosinophilic inflammation, and airway hyperresponsiveness [12]. Even though, allergic asthma is one of the most common chronic inflammatory disorders of the airways in children and adults. However, it is not completely curable yet. Airway inflammation in asthma is associated with T-cell immune response in which Th2 cell derived cytokines including interleukins (IL)-1β, IL-4, IL-5, IL-6 and tumor necrosis factor α (TNF-α) are thought to contribute to eosinophil recruitment, mucus hypersecretion, and airway hyperresponsiveness [345] by controlling the key process of IgE production, the growth of mast cells and the differentiation and activation of mast cells and eosinophil [678]. Mast cells are widely known to contribute to the development of allergic airway disease. Mast cells are tissue cells that are located preferentially at the host-environment interface and in proximity to blood vessels [9]. Mast cells are known mainly for their being involved in mediating various harmful inflammatory reactions and can be activated to release potent mediators by antibody-dependent mechanisms, and can respond to very low dose of specific antigen [101112].
Scutellaria baicalensis Georgi is one of the most popular and multi-purpose herbal medicines or medicinal plants used in oriental countries including China, Japan, and Korea to treat inflammation, allergy, and bacterial and viral infections [131415]. Recently, investigations have shown that S. baicalensis has beneficial properties such as anti-oxidative effect [16], and inhibits anti-dinitrophenyl IgE-medicated anaphylactic reactions and compound 48/80-induced histamine release and calcium uptake into rat peritoneal mast cells (RPMC) [1517]. The flavonoids such as baicalein and wogonin isolated from S. baicalensis have also various biological activities such as anti-oxidative, anti-inflammatory and anti-allergic effects [181920212223]. However, despite studies on anti-allergic effects of S. baicalensis have progressed extensively, little is known about the detailed effects of baicalein, wogonin, and S. baicalensis ethanol extract on ovalbumin (OVA)-induced Th2 cytokine-dependent murine asthma model and compound 48/80-induced systemic anaphylaxis in vivo and compound 48/80-induced mast cell activation in vitro.
In this study, we evaluated the levels of IgE, OVA-specific IgE and IgG1, IgG2a and balance of Th1/Th2 cytokines of OVA-induced murine asthma model in vivo to reveal the anti-asthmatic effect of S. baicalensis ethanol extract and its major flavonoids, baicalein, and wogonin. To further prove the effects of them on mast cell-mediated immune responses, the inhibitory effect of them on compound 48/80-induced systemic anaphylaxis and mast cell activation was evaluated focusing on the histamine release from mast cells.
OVA (grade VI), baicalein, and wogonin were purchase from Sigma-Aldrich (St, Louis, MO, USA). S. baicalensis ethanol extract was provided from the Korea Food Research Institute (KFRI-SL-101). The ethanol extract was obtained by microwave extraction in 70% ethanol for 5 minutes, and concentrated under vacuum in a rotary evaporator. The concentrated extract was lyophilized and dissolved in saline prior to use.
Six-week-old male BALB/c mice for a murine asthma model and 8-week-old male Sprague-Dawley rats for acquirement of RPMC were purchased from Damool Science (Daejeon, Korea). These animals were housed in an air-conditioned room with a 12-hour light/dark cycle. All animal experiments were performed in accordance with the guidelines for Animal Care and Use of Chonbuk National University Laboratory Animal Center.
Mice were divided into 6 groups according to treatment: (1) saline as a vehicle control, (2) OVA-induced asthma mice, (3) 1.25 mg/kg/day dexamethasone as reference drug for positive control, (4) 10 mg/kg/day baicalein, (5) 10 mg/kg/day wogonin, and (6) 200 mg/kg/day S. baicalensis ethanol extract in OVA-induced asthma mice. Mice of groups 2, 3, 4, 5, and 6 were immunized by intraperitoneal injection of 50 µg OVA with 1 mg Imject Alum (Thermo Scientific, Rockford, IL, USA) in a total volume of 200 µl on days 1 and by intraperitoneal injection of 50 µg OVA without Alum on 14. On days 27, 28, and 29 after the beginning of the sensitization period, these mice were challenged for 30 minutes with an aerosol of 5% (wt/vol) OVA in saline using ultrasonic nebulization (NE-U12, Omron Crop., Tokyo, Japan). On days 15 to 26, the treatment groups were also orally treated once daily with baicalein, wogonin, S. baicalensis ethanol extract or dexamethasone. Saline group and OVA-induced asthma group were received only saline. Animals were sacrificed 24 hours after the last challenge on day 30 to investigate the inhibitory effect of baicalein, wogonin, and S. baicalensis ethanol extract.
Twenty-four hours after the final OVA challenge, bronchoalveolar lavage fluid (BALF) was collected by cannulating the upper part of the trachea and lavaging, as described previously [24]. The total number of viable cells in BALF was determined by trypan blue exclusion using a hemocytometer. Differential cell counts were determined with cytospin (Centrifuge 5403, Eppendorf, Hamburg, Germany) preparation, followed by Diff Quik staining (Sysmex Co., Kobe, Japan).
Histopathologic analysis of lung was performed as previously described [24]. Lung were fixed in 10% formalin and embedded in paraffin. Serial 5 µm thickness sections were stained with congo red for eosinophils and inflammatory cells, periodic acid-Schiff (PAS) for goblet cells and mucus and Masson trichrome for collagen fiber deposits.
The levels of Th1 cytokines such as interferon γ (IFN-γ) and Th2-related cytokines such as TNF-α, IL-4, and IL-6 levels in the BALF from each mouse using the appropriate enzyme-linked immunosorbent assay (ELISA; BioSource International, Camarillo, CA, USA) were measured as described earlier [24]. Also OVA-specific IgE, IgG1, and IgG2a were measured according to the manufacturer's instructions.
Mice intraperitoneally received 8 mg/kg body weight (BW) of mast cell degranulator Compound 48/80 or saline as previously described [17]. Baicalein (10 mg/kg BW), wogonin (10 mg/kg BW), or S. baicalensis ethanol extract (100, 200, and 400 mg/kg BW) were dissolved in saline and administered orally at 24, 12, and 1 hour prior to injection of compound 48/80 (n=20/group). Mortality was monitored for 1 hour after induction of anaphylactic shock. After the mortality test, blood was obtained from the heart of each mouse. After centrifugation of blood from mouse heart, the plasma was withdrawn and histamine content was measured by ELISA kit.
RPMC were isolated as previously described [25]. Briefly, rats were anesthetized with ether and peritoneally injected 10 ml of Ca2+-free HEPES-Tyrode buffer, following which the abdomen was gently massaged for approximately 90 seconds. The peritoneal cavity was opened, and fluid was aspirated using a Pasteur pipette. RPMC were purified using a Percoll density gradient, as described in detail elsewhere [25]. RPMC preparation was approximately 95% pure as assessed by toluidine blue staining and at least 98% of these cells were viable as assessed by trypan blue exclusion.
Purified RPMC (1×106 cells/ml) were resuspended in HEPES-Tyrode buffer. The RPMC were pretreated with various concentrations of baicalein, wogonin, or S. baicalensis ethanol extract for 10 minutes at 37℃ and observed for 10 minutes after addition of compound 48/80 (5 µg/ml) under phase contrast microscopy and photographed [25]. The mast cells were classified (×1,000) as follows: (1) extensively degranulated (>50% of the cytoplasmic granules exhibiting fusion, staining alterations and extrusion from the cell), (2) slight to moderately degranulated (10%–50% of the granules exhibiting fusion or discharge), or (3) normal.
RPMC (2×105 cells/well) were pre-incubated with various concentrations of baicalein, wogonin, or S. baicalensis ethanol extract at 37℃ for 10 minutes and then incubated with compound 48/80 (5 µg/ml) for 30 minutes. The cells were separated from the released histamine by centrifugation at 150 ×g for 10 minutes at 4℃. Residual histamine in the cells was released by boiling cells. After centrifugation, histamine content was measured by using ELISA.
As shown in Fig. 1, the numbers of eosinophils, lymphocytes, macrophages and total cells in BALF of mice treated with OVA was significantly increased compared with those of mice treated with saline alone. However, these increases in the numbers of eosinophils, lymphocytes, macrophages, and total cells were significantly reduced by the administration of baicalein, wogonin, or S. baicalensis ethanol extract. Dexamethasone also significantly inhibited the levels of eosinophils and inflammatory cells in BALF.
Histopathological analyses revealed typical pathological features of asthma in OVA-sensitized and -challenged mice. There are increased in the thickness of airway epithelium, the infiltration of eosinophils around bronchioles and blood vessels, the accumulation of mucus and debris in the lumen of bronchioles in OVA-challenged mice compared to control mice (Fig. 2). However, mice treated with baicalein, wogonin, or S. baicalensis ethanol extract showed significantly reductions in the thickening of the airway epithelium, the infiltration of eosinophils around bronchioles and blood vessels, the amount of mucus in the airway lumen (Fig. 2). This increased peribronchial and perivascular lung inflammation was markedly alleviated by the administration of baicalein, wogonin, or S. baicalensis ethanol extract. Dexamethasone also significantly decreased peribronchial and perivascular lung inflammation (Fig. 2).
Mucus hypersecretion which contributes significantly to airflow restriction is accompanied by goblet cell hyperplasia (Fig. 2). In OVA-challenged mice, the accumulation of mucus and debris in the lumen of bronchioles, and hyperplasia of goblet cells in the epithelium of bronchioles significantly increased compared to control mice. The numbers of goblet cells with stained with PAS in airway epithelium was markedly higher in OVA-challenged mice than in control mice (Fig. 2). However, mice treated with baicalein, wogonin, or S. baicalensis ethanol extract significantly inhibited the accumulation of mucus and debris in the lumen of bronchioles, and hyperplasia of goblet cells in the epithelium of bronchioles (Fig. 2). Dexamethasone also significantly suppressed the accumulation of mucus and debris in the lumen of bronchioles, and hyperplasia of goblet cells in the epithelium of bronchioles (Fig. 2).
Collagen deposit among inflammatory cells around bronchiole was significantly increased in the lung of OVA-challenged mice compared to that of control mice (Fig. 2). The treatment of baicalein, wogonin, or S. baicalensis ethanol extract reduced the collagen deposit around bronchiole by OVA. Dexamethasone also inhibited the levels of peribronchial fibrosis.
To investigate the underlying immunoregulatory mechanism of baicalein, wogonin, and S. baicalensis ethanol extract on anti-asthma, we detected serum levels of total IgE and OVA-specific IgE, IgG1 (Th2 related Ig), and IgG2a (Th1 related Ig). As shown in Fig. 3, the levels of total IgE (Fig. 3A), OVA-specific IgE (Fig. 3B), and OVA-specific IgG1 (Fig. 3C) were markedly increased in OVA-challenged mice compared with control mice. The administration of baicalein, wogonin, or S. baicalensis ethanol extract reduced the levels of total IgE and both the OVA-specific IgE and IgG1 in serum. However, neither baicalein, wogonin nor S. baicalensis ethanol extract inhibited the production of OVA-specific IgG2a (Fig. 3D).
Next, to further delineat the anti-asthmatic effects of baicalein, wogonin S. baicalensis ethanol extract, we investigated the levels of Th1/Th2 cytokines including IFN-γ, TNF-α, IL-4, IL-5, and IL-6 that known to regulate IgE-mediated allergy and asthma [2627] in the BALF using appropriate ELISA. The levels of Th1 cytokines such as IFN-γ and IL-12 in BALF were decreased and the levels of Th2 cytokines IL-1β, IL-4, IL-5, and TNF-α in BALF were significantly increased in OVA-challenged compared with control mice (Fig. 4). The decreased levels of IFN-γ and IL-12 were significantly increased and the increased levels of IL-1β, IL-4, IL-5, and TNF-α were reduced by the administration of baicalein, wogonin or S. baicalensis ethanol extract, which were similar to the increment of IFN-γ and IL-12 and the reduction of IL-1β, IL-4, IL-5, and TNF-α in BALF by dexamethasone (Fig. 4).
To investigate the effect of S. baicalensis ethanol extract in anaphylactic shock, we first used the in vivo model of systemic anaphylaxis using compound 48/80. As shown in Fig. 5, the intraperitoneal injection of compound 48/80 (8 mg/kg BW) resulted in 93.33% death of mice. However, oral administration of S. baicalensis ethanol extract (200 to 400 mg/kg BW) reduced compound 48/80-induced mortality in dose-dependent manner (Fig. 5A). Also, baicalein (10 mg/kg BW) and wogonin (10 mg/kg BW) significantly suppressed the compound 48/80-induced mortality (Fig. 5A). The effect of baicalein, wogonin S. baicalensis ethanol extract on compound 48/80-induced plasma histamine release in mice was investigated. The content of plasma histamine in mice treated with compound 48/80 was 315.7±21.8 ng/ml. As shown in Fig. 5B, the inhibition effects on histamine release by oral administration of S. baicalensis ethanol extract was significant at doses of 200 to 400 mg/kg BW. In addition, baicalein and wogonin significantly decreased the plasma histamine release induced by compound 48/80 (Fig. 5B).
Mast cells in asthma have been known to exert their pathophysiological role through histamine release by degranulation [28]. To investigate whether baicalein, wogonin, or S. baicalensis ethanol extract directly suppressed histamine release from RPMC, we used compound 48/80 that has been known as a stimulator the degranulation of RPMC through perturbation of the membrane [29].
Compound 48/80-treated RPMC showed cell swelling, cytoplasmic vacuoles, irregular membrane, and extruded granules near the cell surface and in the surrounding media that is referred as mast cell degranulation (Fig. 6B). The normal RPMC were generally oval or round in shape and were full of many fine granules surrounding a prominent nucleus in their cytoplasm (Fig. 6A). However, pretreatment with baicalein, wogonin or S. baicalensis ethanol extract inhibited RPMC degranulation by compound 48/80 (Fig. 6D–F), were similar to normal RPMC. Dexamethasone also inhibited the mast cell degranulation (Fig. 6C). Baicalein, wogonin, and S. baicalensis ethanol extract might protect the lipid membrane via the inhibition of the perturbation induced by compound 48/80 (Fig. 6G). Moreover, baicalein, wogonin, and S. baicalensis ethanol extract diminished compound 48/80-induced histamine release from RPMC at concentrations used in this study (Fig. 6H). These results suggested that baicalein, wogonin, and S. baicalensis ethanol extract may alleviate compound 48/80-induced histamine release from mast cells by the suppression of mast cell degranulation.
The radix of S. baicalensis has been used as a remedy in the traditional medicine due to its powerful anti-inflammatory activity and low toxicity to humans. S. baicalensis contains various components, and more than 60 flavonoids have been identified from different sources of S. baicalensis [3031]. Many reports show that baicalein and wogonin are the principal active components of S. baicalensis and have potent anti-cancer and anti-inflammatory effects [18192021222331]. Here, compared with their anti-inflammatory effects, we demonstrated the anti-allergic and anti-anaphylactic effects of these flavonoids using OVA-induced asthma and compound 48/80-induced systemic anaphylaxis mice models and studied with mast cell-mediated responses in vitro.
In this study, we demonstrated the inhibitory effect of baicalein, wogonin and S. baicalensis ethanol extract on airway inflammation using a classical asthmatic murine model induced by OVA. OVA-induced allergic asthma is well known as a disease that results from chronic airway inflammation typically associated with the infiltration of eosinophils, lymphocytes, and macrophages into the bronchial lumen [222324]. Eosinophils are markers of allergic airway inflammation in asthma and play a critical role in the pathogenesis of asthma [2932]. It has been known that there are a correlation between pulmonary eosinophilia and asthma, as well as a correlation with the level of eosinophils in the BALF [242932]. Similar to some studies [1823], baicalein, wogonin, and S. baicalensis ethanol extract significantly inhibited the airway inflammation around bronchi and blood vessels, the hyperplasia of goblet cells, the deposit of collagen fibers, the infiltration of inflammatory cells especially eosinophils in the OVA-induced asthmatic lung tissues. Also, we observed baicalein, wogonin, and S. baicalensis ethanol extract resulted in a significant decrease in the number of eosinophils and lymphocytes in BALF, these cells could be the targets of baicalein, wogonin and S. baicalensis.
In murine asthma model, OVA challenges induced a significant increase in the total serum IgE and OVA-specific IgG1 and BALF IgE [3334]. Our present study showed oral administration of baicalein, wogonin, and S. baicalensis ethanol extract markedly reduced the OVA-induced total IgE and OVA-specific IgE in serum, which was similar to the reduction in dexamethasone treated mice. These results suggest that baicalein and wogonin have therapeutic potential on the allergic asthma that developed in an IgE-dependent manner.
Airway inflammation in asthma is associated with T-cell immune response in which Th2 cell derived cytokines including IL-1β, IL-4, IL-5, and TNF-α are thought to contribute to eosinophil recruitment, mucus hypersecretion, and airway hyperresponsiveness [345] by controlling the key process of IgE production, the growth of mast cells and the differentiation and activation of mast cells and eosinophil [678]. Asthma and inflammation are associated with enhanced production of IL-4 and decreased production of IFN-γ. Compared with Th2 cytokines, Th1 cytokines such as IFN-γ and IL-12 are associated to antagonism of Th2 cell responses and IgE synthesis to restrain the progress of asthma. In physiological condition, immune responses of Th1 and Th2 cytokines maintain dynamic balance. Whenever this balance is disturbed, diseases will occur [35]. Accordingly, examination of levels of Th1/Th2 cytokines is important index in the evaluation of asthma. To confirm effects of baicalein, wogonin, and S. baicalensis ethanol extract on Th1/Th2 cytokines further, we examined the production of Th2 cytokines such as TNF-α, IL-1β, IL-4, and IL-5, and Th1 cytokines such as IFN-γ and IL-12. We showed here that administration of baicalein, wogonin, and S. baicalensis ethanol extract markedly reduced the production of IL-4 and IL-5, and also counteracted the OVA-induced production of IL-1β and TNF-α, pro-inflammatory cytokines, induced by OVA challenges. Consistent with reduction of the levels of Th2 cytokines, the infiltration of eosinophils were significantly suppressed in the BALF and lung tissues after treatment of baicalein or wogonin. However, oral administration of baicalein, wogonin, and S. baicalensis ethanol extract enhanced the production of Th1 cytokines including IFN-γ and IL-12. From these results, the imbalanced Th1/Th2 cytokines are confirmed to be a key factor for asthma that increases airway inflammation. These results suggest that baicalein and wogonin could regulate the balance of Th1/Th2 cytokines by suppression of the development of airway inflammation via shifting from a Th2 to Th1 response in the OVA-induced asthma. However, detailed molecular and cellular mechanisms still remain to be elucidated, and we intend to examine these mechanisms in our future study.
Mast cell activation and the release of stored mediators and cytokines including histamine and IL-4 are key events in asthmatic airway [36]. Histamine has been known one of the most important chemical mediators of allergy and inflammation [3738], and plays an important role in eliciting nasal symptoms of allergic rhinitis such as sneezing and itch [39]. Recently, antihistamines have been used as a therapy for asthma to evoke acute bronchodilation [40]. However, these antihistamines tend to cause side effects and reduce receptor binding affinity and T-cell response [41]. Due to these reasons, herbal medicines derived from plant and herbs are focusing more and more as therapies for allergic disorders. We used compound 48/80 that has been known as a stimulator and inducer histamine release from mast cells and investigated whether baicalein, wogonin, and S. baicalensis ethanol extract directly has anti-histamine effect to evaluate the therapeutic efficacy of baicalein, wogonin, and S. baicalensis ethanol extract in OVA-induced allergic asthma via suppression of histamine release from mast cells. Baicalein, wogonin, and S. baicalensis ethanol extract inhibited compound 48/80-induced systemic anaphylaxis and plasma histamine release in mice. Also, baicalein, wogonin, and S. baicalensis ethanol extract significantly inhibited compound 48/80-induced mast cell degranulation and histamine release from RPMC.
In the present study, we demonstrated that baicalein, wogonin, and S. baicalensis ethanol extract downregulate OVA-induced Th2 type airway inflammation and compound 48/80-induced systemic anaphylaxis and mast cell activation, and upregulate Th1 cytokines, especially IFN-γ and IL-12 production. These data suggest that baicalein, wogonin and S. baicalensis ethanol extract may have preventive and therapeutic activities for Th2 type or mast cell-mediated allergic diseases.
Figures and Tables
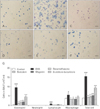 | Fig. 1Effect of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on infiltration of inflammatory cells (A–F) and differential cellular components and total cells (G) in bronchoalveolar lavage fluid (BALF) of mice induced by ovalbumin (OVA) sensitization and challenge. (A) Control. (B) Saline. (C) Dexamethasone 1.5 mg/kg. (D) Baicalein 10 mg/kg. (E) Wogonin 10 mg/kg. (F) S. baicalensis ethanol extract 200 mg/kg. Values are presented as the mean±SD (n=6 per group). Data were analyzed using ANOVA followed by Student's t test. ###P<0.001, #P<0.05, significantly different from the value of saline group. ***P<0.001, *P<0.05, significantly different from the value of OVA group. Results are presented as the mean±SD (n=6 per group). |
 | Fig. 2Effect of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on ovalbumin (OVA)-induced histopathological changes. Mice were sensitized on days 1 and 14, and challenged on days 27, 28, and 29 by OVA. On days 15 to 26, the treatment groups were also orally treated once daily with saline, dexamethasone 1.5 mg/kg, baicalein 10 mg/kg, wogonin 10 mg/kg, and S. baicalensis ethanol extract 200 mg/kg. Lung tissues from each group were stained with congo red for eosinophils, periodic acid-Schiff (PAS) for goblet cells (white arrows) and mucus (white asterisk) and Masson trichrome for collagen fiber deposits (black arrows). Only a representative picture is shown for each group. Scale bars=100 µm. |
 | Fig. 3Effect of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on the serum levels of total IgE (A), ovalbumin (OVA)-specific IgE (B), OVA-specific IgG1 (C), and OVA-specific IgG2 (D). Data were analyzed using ANOVA followed by Student's t test. ###P<0.001, significantly different from the value of saline group. ***P<0.001, **P<0.01, *P<0.05, significantly different from the value of OVA group. Results are presented as the mean±SD (n=6 per group). |
 | Fig. 4Effect of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on the levels of Th1/Th2 cytokines in bronchoalveolar lavage fluid. (A) Tumor necrosis factor α (TNF-α). (B) Interleukin (IL)-1β. (C) IL-4. (D) IL-5. (E) IL-12. (F) Interferon γ (IFN-γ). Data were analyzed using ANOVA followed by Student's t test. ###P<0.001, ##P<0.01, #P<0.05, significantly different from the value of saline group. ***P<0.001, **P<0.01, *P<0.05, significantly different from the value of ovalbumin (OVA) group. Results are presented as the mean±SD (n=6 per group). |
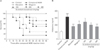 | Fig. 5Inhibitory effect of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on compound 48/80-induced systemic anaphylaxis. Five groups of mice (n=7/group) were orally administered saline, baicalein (10 mg/kg body weight [BW]), wogonin (10 mg/kg BW) or S. baicalensis ethanol extract (100, 200, and 400 mg/kg BW), at 24, 12, and 1 hour prior to injection of compound 48/80. The compound 48/80 was intraperitoneally given to mice. (A) Survival rates (%) were monitored for 1 hour after induction of anaphylactic shock. (B) Histamine concentrations in serum were measured by enzyme-linked immunosorbent assay. Each data represents the mean±SD (n=7 per group). Data were analyzed using ANOVA followed by Student's t test. **P<0.01, ***P<0.001, significantly different from the value of saline treated group. Results are presented as the mean±SD. ###P<0.001, significantly different from the value of saline group. |
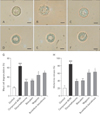 | Fig. 6Effect of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on rat mast cell degranulation and histamine release from rat peritoneal mast cells (RPMCs). RPMCs were preincubated with dexamethasone, baicalein, wogonin, or S. baicalensis extract at 37℃ for 10 minutes before incubation with compound 48/80. The RPMCs were micrographed within 10 minutes after the addition of saline or compound 48/80 by using inverted phase contrast microscopy. (A) Saline as a control. (B) Compound 48/80 5 µg/ml. (C) Dexamethasone 1.5 mg/ml. (D) Baicalein 10 mg/ml. (E) Wogonin 10 mg/ml. (F) S. baicalensis ethanol extract 200 mg/ml. Scale bars=10 µm (A–F). (G) Quantitation of mast cell degranulation. (H) Histamine release (%). Data were analyzed using ANOVA followed by Student's t test. ###P<0.001, significantly different from the value of saline group. ***P<0.001, **P<0.01, *P<0.05, significantly different from the value of ovalbumin group. Results are presented as the mean±SD (n=6 per group). |
Acknowledgements
This study was supported by research grants (E0121304-05) from the Korea Food Research Institute and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A303857).
References
1. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014; 7:53–65.
2. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56.
3. Kim YS, Choi SJ, Choi JP, Jeon SG, Oh S, Lee BJ, Gho YS, Lee CG, Zhu Z, Elias JA, Kim YK. IL-12-STAT4-IFN-gamma axis is a key downstream pathway in the development of IL-13-mediated asthma phenotypes in a Th2 type asthma model. Exp Mol Med. 2010; 42:533–546.
4. Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015; 7:301ra129.
5. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395.
6. Troy NM, Hollams EM, Holt PG, Bosco A. Differential gene network analysis for the identification of asthma-associated therapeutic targets in allergen-specific T-helper memory responses. BMC Med Genomics. 2016; 9:9.
7. Punnonen J, Aversa G, Cocks BG, de Vries JE. Role of interleukin-4 and interleukin-13 in synthesis of IgE and expression of CD23 by human B cells. Allergy. 1994; 49:576–586.
8. Abdelmotelb AM, Rose-Zerilli MJ, Barton SJ, Holgate ST, Walls AF, Holloway JW. Alpha-tryptase gene variation is associated with levels of circulating IgE and lung function in asthma. Clin Exp Allergy. 2014; 44:822–830.
9. Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett. 2016; 178:10–14.
10. Dahlin JS, Hallgren J. Mast cell progenitors: origin, development and migration to tissues. Mol Immunol. 2015; 63:9–17.
11. Galli SJ. The mast cell-IgE paradox: from homeostasis to anaphylaxis. Am J Pathol. 2016; 186:212–224.
12. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014; 62:698–738.
13. Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol. 2009; 125:286–290.
14. Mir-Palomo S, Nácher A, Díez-Sales O, Ofelia Vila Busó MA, Caddeo C, Manca ML, Manconi M, Fadda AM, Saurí AR. Inhibition of skin inflammation by baicalin ultradeformable vesicles. Int J Pharm. 2016; 511:23–29.
15. Jung HS, Kim MH, Gwak NG, Im YS, Lee KY, Sohn Y, Choi H, Yang WM. Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J Ethnopharmacol. 2012; 141:345–349.
16. Kim OS, Seo CS, Kim Y, Shin HK, Ha H. Extracts of Scutellariae Radix inhibit low-density lipoprotein oxidation and the lipopolysaccharide-induced macrophage inflammatory response. Mol Med Rep. 2015; 12:1335–1341.
17. Choi YH, Han EH, Chai OH, Kim YK, Kim HT, Song CH. Scutellaria baicalensis inhibits mast cell-mediated anaphylactic reactions. Korean J Phys Anthropol. 2010; 23:217–227.
18. Liu T, Dai W, Li C, Liu F, Chen Y, Weng D, Chen J. Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J Nat Prod. 2015; 78:3049–3057.
19. He X, Wei Z, Zhou E, Chen L, Kou J, Wang J, Yang Z. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int Immunopharmacol. 2015; 28:470–476.
20. Nakajima T, Imanishi M, Yamamoto K, Cyong JC, Hirai K. Inhibitory effect of baicalein, a flavonoid in Scutellaria root, on eotaxin production by human dermal fibroblasts. Planta Med. 2001; 67:132–135.
21. Mabalirajan U, Ahmad T, Rehman R, Leishangthem GD, Dinda AK, Agrawal A, Ghosh B, Sharma SK. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PLoS One. 2013; 8:e62916.
22. Ryu EK, Kim TH, Jang EJ, Choi YS, Kim ST, Hahm KB, Lee HJ. Wogonin, a plant flavone from Scutellariae radix, attenuated ovalbumin-induced airway inflammation in mouse model of asthma via the suppression of IL-4/STAT6 signaling. J Clin Biochem Nutr. 2015; 57:105–112.
23. Shin HS, Bae MJ, Choi DW, Shon DH. Skullcap (Scutellaria baicalensis) extract and its active compound, wogonin, inhibit ovalbumin-induced Th2-mediated response. Molecules. 2014; 19:2536–2545.
24. Chai OH, Han EH, Lee HK, Song CH. Mast cells play a key role in Th2 cytokine-dependent asthma model through production of adhesion molecules by liberation of TNF-α. Exp Mol Med. 2011; 43:35–43.
25. Chai OH, Shon DH, Han EH, Kim HT, Song CH. Effects of Anemarrhena asphodeloides on IgE-mediated passive cutaneous anaphylaxis, compound 48/80-induced systemic anaphylaxis and mast cell activation. Exp Toxicol Pathol. 2013; 65:419–426.
26. Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016; 28:163–171.
27. Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014; 85:36–42.
28. Winbery SL, Lieberman PL. Histamine and antihistamines in anaphylaxis. Clin Allergy Immunol. 2002; 17:287–317.
29. Busse WW, Rosenwasser LJ. Mechanisms of asthma. J Allergy Clin Immunol. 2003; 111:3 Suppl. S799–S804.
30. Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci. 2004; 812:277–290.
31. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009; 35:57–68.
32. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.
33. Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness: a murine model. Allergy. 1999; 54:297–305.
34. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005; 115:459–465.
35. Guo HW, Yun CX, Hou GH, Du J, Huang X, Lu Y, Keller ET, Zhang J, Deng JG. Mangiferin attenuates TH1/TH2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS One. 2014; 9:e100394.
36. Moiseeva EP, Bradding P. Mast cells in lung inflammation. Adv Exp Med Biol. 2011; 716:235–269.
37. White MV. The role of histamine in allergic diseases. J Allergy Clin Immunol. 1990; 86(4 Pt 2):599–605.
38. Rafferty P, Beasley R, Holgate ST. The contribution of histamine to immediate bronchoconstriction provoked by inhaled allergen and adenosine 5' monophosphate in atopic asthma. Am Rev Respir Dis. 1987; 136:369–373.
39. Taylor-Clark TE. Insights into the mechanisms of histamine-induced inflammation in the nasal mucosa. Pulm Pharmacol Ther. 2008; 21:455–460.
40. Nelson HS. Prospects for antihistamines in the treatment of asthma. J Allergy Clin Immunol. 2003; 112:4 Suppl. S96–S100.
41. Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993; 151:3460–3466.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download