Abstract
Figures and Tables
Fig. 1
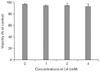
Fig. 2
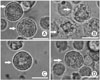
Fig. 3

Fig. 4

Fig. 5

Table 1
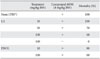
Groups of mice (n=10/group) were orally administered with 300 µl of PBS or drugs {LA or disodium cromoglycate (DSCG)} at 24, 12 and 1 h before the injection of compound 48/80. The compound 48/80 solution was intraperitoneally given to the group of mice. Mortality (%) within 1 h following compound 48/80 injection was presented as the number of dead mice ×100/total number of experimental mice. *PBS, phosphate-buffered saline.
Table 2
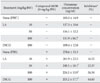
LA or disodium cromoglycate (DSCG) was administered orally at 24, 12 and 1 h prior to the injection of compound 48/80. The compound 48/80 solution was intraperitoneally given to the group of mice. Each histamine concentration represents the mean±S.E.M. of five independent experiments. *Inhibition (%) = {1-(T-B)/(C-N)} ×100. Control (C): compound 48/80 (+), LA (-); Normal (N): compound 48/80 (-), LA (-); Test (T): compound 48/80 (+), LA (+); Blank (B): compound 48/80 (-), LA (+). †PBS, phosphate-buffered saline. ‡P<0.05, significantly different from the control value. DSCG (100 mg/kg) was used as a typical antihistamine control drug.
Table 3
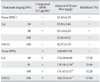
LA or disodium cromoglycate (DSCG) was administered orally at 24, 12 and 1 h before the injection of compound 48/80. Twenty microliters of compound 48/80 (0.5 µg/site) were intradermally injected to the backs of mice. Each amount of Evans blue represents the mean±S.E.M. of five independent experiments. *Inhibition (%) = {1-(T-B)/(C-N)} ×100. Control (C): compound 48/80 (+), LA (-); Normal (N): compound 48/80 (-), LA (-); Test (T): compound 48/80 (+), LA (+); Blank (B): compound 48/80 (-), LA (+). †PBS, phosphate-buffered saline. ‡P<0.05, significantly different from the control value. DSCG (100 mg/kg) was used as a typical antihistamine control drug.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download