1. Retzepi M, Donos N. Guided bone regeneration: biological principle and therapeutic applications. Clin Oral Implants Res. 2010; 21:567–576. PMID:
20666785.

2. Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006; 15:8–17. PMID:
16569956.

3. Rezende ML, Consolaro A, Sant'Ana AC, Damante CA, Greghi SL, Passanezi E. Demineralization of the contacting surfaces in autologous onlay bone grafts improves bone formation and bone consolidation. J Periodontol. 2014; 85:e121–e129. PMID:
24171500.

4. Pérez M, Fernández I, Márquez D, Bretaña RM. Use of N-butyl-2-cyanoacrylate in oral surgery: biological and clinical evaluation. Artif Organs. 2000; 24:241–243. PMID:
10759649.
5. Choi BH, Kim BY, Huh JY, Lee SH, Zhu SJ, Jung JH, et al. Cyanoacrylate adhesive for closing sinus membrane perforations during sinus lifts. J Craniomaxillofac Surg. 2006; 34:505–509. PMID:
17157515.

6. Rezende ML, Cunha PO, Damante CA, Santana AC, Greghi SL, Zangrando MS. Cyanoacrylate adhesive as an alternative tool for membrane fixation in guided tissue regeneration. J Contemp Dent Pract. 2015; 16:512–518. PMID:
26323456.
7. Baş B, Ozden B, Bekçioğlu B, Sanal KO, Gülbahar MY, Kabak YB. Screw fixation is superior to N-butyl-2-cyanoacrylate in onlay grafting procedure: a histomorphologic study. Int J Oral Maxillofac Surg. 2012; 41:537–543. PMID:
22113114.

8. Chang YY, Dissanayake S, Yun JH, Jung UW, Kim CS, Park KJ, et al. The biological effect of cyanoacrylate-combined calcium phosphate in rabbit calvarial defects. J Periodontal Implant Sci. 2011; 41:123–130. PMID:
21811687.

9. Lu Q, Danner E, Waite JH, Israelachvili JN, Zeng H, Hwang DS. Adhesion of mussel foot proteins to different substrate surfaces. J R Soc Interface. 2013; 10:20120759. PMID:
23173195.

10. Waite JH. Mussel adhesion - essential footwork. J Exp Biol. 2017; 220:517–530. PMID:
28202646.

11. Martinez Rodriguez NR, Das S, Kaufman Y, Wei W, Israelachvili JN, Waite JH. Mussel adhesive protein provides cohesive matrix for collagen type-1α. Biomaterials. 2015; 51:51–57. PMID:
25770997.

12. Waite JH. Adhesion a la moule. Integr Comp Biol. 2002; 42:1172–1180. PMID:
21680402.

13. Dove J, Sheridan P. Adhesive protein from mussels: possibilities for dentistry, medicine, and industry. J Am Dent Assoc. 1986; 112:879. PMID:
3458804.
14. Castillo JJ, Shanbhag BK, He L. Comparison of natural extraction and recombinant mussel adhesive proteins approaches. In : Puri M, editor. Food bioactives. Basel: Springer;2017. p. 111–135.
15. Yu M, Hwang J, Deming TJ. Role of L-3, 4-dihydroxyphenylalanine in mussel adhesive proteins. J Am Chem Soc. 1999; 121:5825–5826.
16. Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006; 103:12999–13003. PMID:
16920796.

17. Cha HJ, Hwang DS, Lim S, White JD, Matos-Perez CA, Wilker JJ. Bulk adhesive strength of recombinant hybrid mussel adhesive protein. Biofouling. 2009; 25:99–107. PMID:
18985469.

18. Hwang DS, Gim Y, Yoo HJ, Cha HJ. Practical recombinant hybrid mussel bioadhesive fp-151. Biomaterials. 2007; 28:3560–3568. PMID:
17507090.

19. Grande DA, Pitman MI. The use of adhesives in chondrocyte transplantation surgery. Preliminary studies. Bull Hosp Jt Dis Orthop Inst. 1988; 48:140–148. PMID:
2854480.
20. Hong JM, Kim BJ, Shim JH, Kang KS, Kim KJ, Rhie JW, et al. Enhancement of bone regeneration through facile surface functionalization of solid freeform fabrication-based three-dimensional scaffolds using mussel adhesive proteins. Acta Biomater. 2012; 8:2578–2586. PMID:
22480947.

21. Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials. 2012; 33:7972–7983. PMID:
22902057.

22. Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, et al. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010; 40:180–187. PMID:
20827327.

23. Duarte A, Coelho J, Bordado J, Cidade M, Gil M. Surgical adhesives: systematic review of the main types and development forecast. Prog Polym Sci. 2012; 37:1031–1050.

24. Tseng YC, Hyon SH, Ikada Y. Modification of synthesis and investigation of properties for 2-cyanoacrylates. Biomaterials. 1990; 11:73–79.

25. Eiferman RA, Snyder JW. Antibacterial effect of cyanoacrylate glue. Arch Ophthalmol. 1983; 101:958–960. PMID:
6683097.

26. Al-Belasy FA, Amer MZ. Hemostatic effect of n-butyl-2-cyanoacrylate (histoacryl) glue in warfarin-treated patients undergoing oral surgery. J Oral Maxillofac Surg. 2003; 61:1405–1409. PMID:
14663804.

27. Kutcher MJ, Ludlow JB, Samuelson AD, Campbell T, Pusek SN. Evaluation of a bioadhesive device for the management of aphthous ulcers. J Am Dent Assoc. 2001; 132:368–376. PMID:
11258094.

28. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81:672–676. PMID:
3362985.

29. Weng D, Hürzeler MB, Quiñones CR, Ohlms A, Caffesse RG. Contribution of the periosteum to bone formation in guided bone regeneration. A study in monkeys. Clin Oral Implants Res. 2000; 11:546–554. PMID:
11168248.
30. Yang JW, Park HJ, Yoo KH, Chung K, Jung S, Oh HK, et al. A comparison study between periosteum and resorbable collagen membrane on iliac block bone graft resorption in the rabbit calvarium. Head Face Med. 2014; 10:15. PMID:
24886656.

31. Jung UW, Lee JS, Lee G, Lee IK, Hwang JW, Kim MS, et al. Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs. J Periodontal Implant Sci. 2013; 43:64–71. PMID:
23678389.

32. Park JY, Jung IH, Kim YK, Lim HC, Lee JS, Jung UW, et al. Guided bone regeneration using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-cross-linked type-I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomater Res. 2015; 19:15. PMID:
26331084.

33. Lim HC, Song KH, You H, Lee JS, Jung UW, Kim SY, et al. Effectiveness of biphasic calcium phosphate block bone substitutes processed using a modified extrusion method in rabbit calvarial defects. J Periodontal Implant Sci. 2015; 45:46–55. PMID:
25932338.

34. Minabe M, Kodama T, Kogou T, Tamura T, Hori T, Watanabe Y, et al. Different cross-linked types of collagen implanted in rat palatal gingiva. J Periodontol. 1989; 60:35–43. PMID:
2921711.

35. Iglhaut J, Aukhil I, Simpson DM, Johnston MC, Koch G. Progenitor cell kinetics during guided tissue regeneration in experimental periodontal wounds. J Periodontal Res. 1988; 23:107–117. PMID:
2967362.

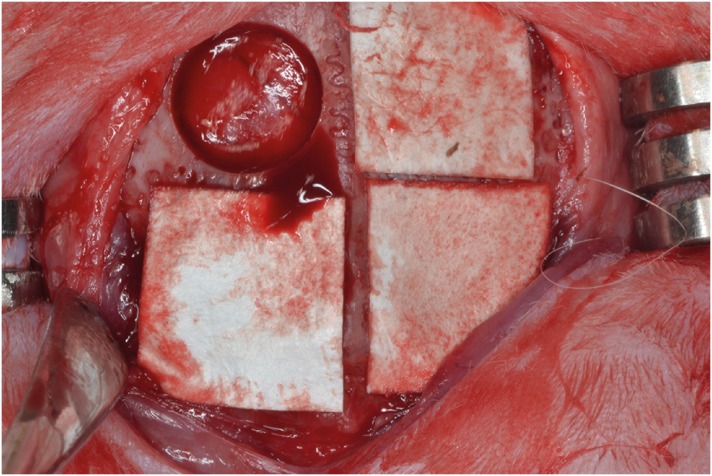
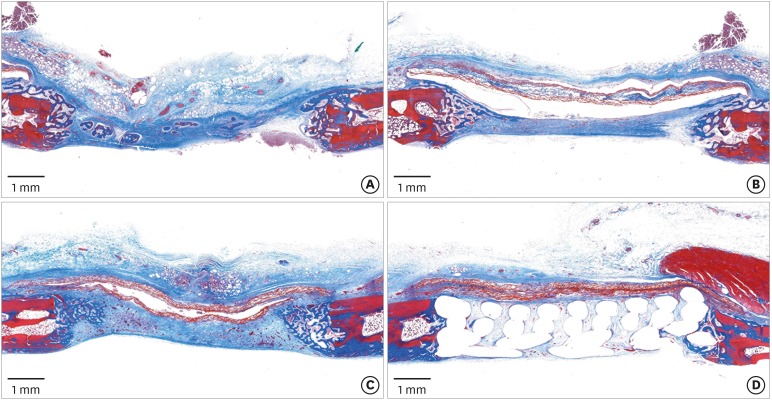
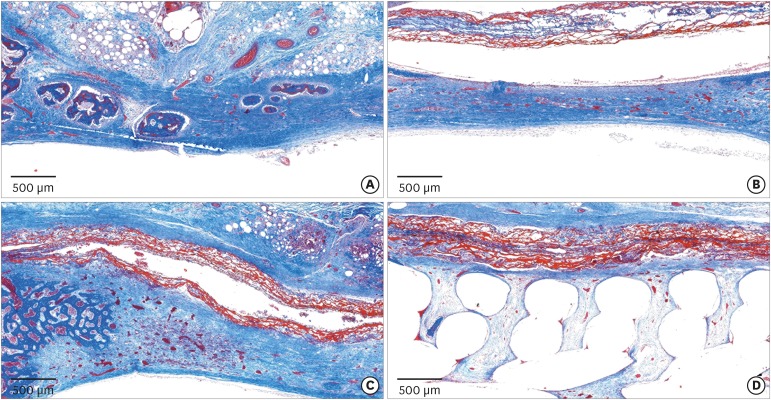
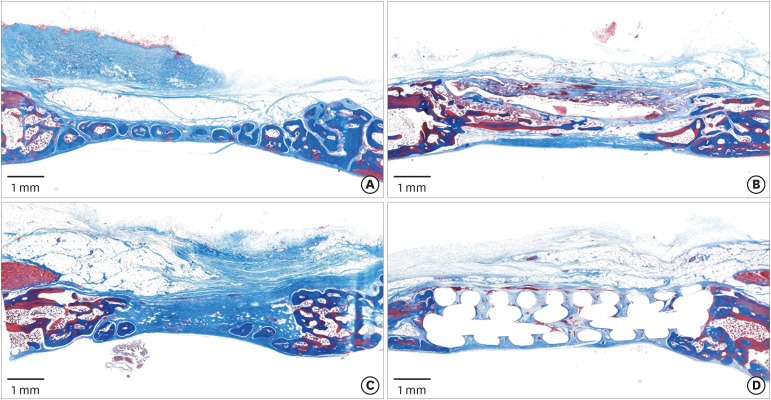




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download