Abstract
Osmotic demyelination syndrome is a demyelinating disorder associated with rapid correction of hyponatremia. But, it rarely occurs in acute hypernatremia, and it leads to permanent neurologic symptoms and is associated with high mortality. A 44-year-old woman treated with alternative medicine was admitted with a history of drowsy mental status. Severe hypernatremia (197mEq/L) with hyperosmolality (415mOsm/kgH2O) was evident initially and magnetic resonance imaging revealed a high signal intensity lesion in the pons, consistent with central pontine myelinolysis. She was treated with 0.45% saline and 5% dextrose water and intravenous corticosteroids. Serum sodium normalized and her clinical course gradually improved. Brain lesion of myelinolysis also improved in a follow-up imaging study. This is the first report of a successful treatment of hypernatremia caused by iatrogenic salt intake, and it confirms the importance of adequate fluid supplementation in severe hypernatremia.
Severe hypernatremia, defined as a rise in the serum sodium concentration to a value exceeding 160mEq/L, leads to the failure of the brain defense mechanism to adapt itself to change, and therefore, it is associated with high mortality due to encephalopathy123). Osmotic demyelination syndrome (ODS), which includes both central pontine myelinolysis (CPM) and extrapontine myelinolysis (EPM), is one of the encephalopathies that result from extreme fluctuations in serum sodium concentration and plasma osmolality4). Only a few case reports have documented ODS, and it usually occurs during rapid correction of hyponatremia2). ODS due to hypernatremia have been reported in 30 adult patients who developed acute hypernatremia due to various etiologies; the anatomical structure most frequently affected by osmotic myelinolysis is central pons56). Here we present a case of osmotic myelinolysis, which was successfully treated by adequate fluid supplementation.
A 44-year-old woman was admitted to our hospital with a drowsy mental status since a few days ago. She had a history of ulcerative colitis that was treated with medication (oral prednisolone 35mg/day, mesalazine 3,000 mg/day). Two weeks prior to admission, she had discontinued the steroid treatment and wanted to be treated with alternative medicine that included sodium chloride, multivitamin, and selenium with discontinuation of her regular diet.
Clinically, she was severely dehydrated due to frequent watery diarrhea. She was hypotensive and her blood pressure was 80/50mmHg. On initial examination, she was stuporous and slightly confused, and she responded only to painful stimuli and had weakness in both arms and legs. A hypoactive deep tendon reflex was present, but there were no other focal neurological deficits or neck stiffness.
Initial blood laboratory test results showed a sodium level of 197mEq/L (range, 135-146mEq/L), potassium level of 2.9mEq/L (range, 3.5-5.3mEq/L), chloride level of 159mEq/L (range, 99-108mEq/L), blood urea nitrogen level of 96mg/dL (range, 8-19mg/dL), creatinine level of 1.56mg/dL (range, 0.67-1.17mg/dL), serum osmolality of 415mOsm/kgH2O(range, 280-300mOsm/kgH2O), ACTH level of 3.99 pg/mL (range, 7.2-63.3 pg/mL), and serum cortisol level of 30.84 ug/dL (range, 4.3-22.4 ug/dL). Urinalysis showed a specific gravity of 1.030, osmolality of 885mOsm/kgH2O, urine sodium level of 12.0mEq/L, and urine creatinine level of 128.1mg/dL.
The patient was admitted to the medical intensive care unit where intravenous fluids were rapidly administered and serial monitoring of electrolytes and osmolality was started. There was no evidence of adrenal insufficiency, but we intravenously administered dexamethasone 5mg and hydrocortisone 100mg/day considering her history of steroid use for protection from adrenal insufficiency. Immediate treatment with 0.45% saline and 5% dextrose water was started along with modification of the rate and concentration so that the fall in serum sodium level did not exceed 0.5mEq/L/hour. Despite our effort to avoid very rapid correction of hypernatremia, the correction rate exceeded 1mEq/L/hour (Fig. 1, Table 1).
Six days after admission, the serum sodium level was 149mEq/L and she had partially recovered her mental status, especially her early depression of consciousness level; however, weakness in all extremities persisted. Diffusion-weighted (DW) magnetic resonance imaging (MRI) performed on hospital day 6 revealed multifocal, symmetric, diffusion restriction within the lateral pons, thalami, and splenium of the corpus callosum(Fig. 2). However, there were no abnormal signal intensity changes on fluid-attenuated inversion recovery (FLAIR) images. Considering the clinical and radiologic findings, despite the unusual pattern in the pons, our diagnosis was osmotic demyelination syndrome involving both the lateral pontine and extrapontine sites associated with rapid correction of hypernatremia. The patient was closely observed and was treated with corticosteroid therapy and conservative management including nutritional support.
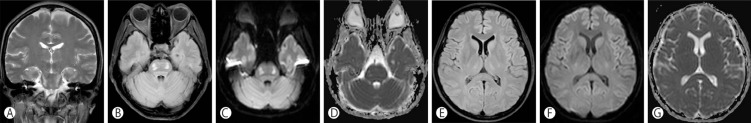
Follow-up MRI performed on hospital day 30 revealed high signal intensity changes in the lateral pons and bilateral thalami on FLAIR images, which were pseudonormalized on DW images (Fig. 3). The previously noted corpus callosum lesion had resolved on FLAIR and DW images. The patient's mental status and motor weakness were improved, and she was discharged to a rehabilitation hospital at hospital day 49. She remained free of neurologic symptoms during the 6-month follow-up period, and the serum sodium concentration had stabilized.
Hypernatremia develops due to a disturbance of water homeostasis involving excessive water loss or excessive solute gain. An exogenous sodium overload can occur accidentally in patients admitted to the intensive care unit due to iatrogenic hypertonic saline infusion, but it occurred in only one patient following deliberate sodium chloride ingestion as per the reports7).
ODS usually occurs during a rapid correction of hyponatremia, but it rarely occurs in severe hypernatremia2). The mechanism of ODS in hypernatremia is poorly described and understood. Rapid correction of hypernatremia and rapid change from normal serum sodium levels to marked hypernatremia may cause myelinolysis in humans, based on supporting data from animal studies8).
ODS includes CPM and EPM, which is evident on computed tomography. However, MRI is the imaging technique of choice because of its greater sensitivity and superior capacity for demonstration of the lesions10). The typical radiologic feature of CPM is a trident-shaped, symmetric hyperintense lesion in the central pons with sparing of the periphery on T2-weighted MRI and hypointensity on the T1-weighted image11). In cases of EPM, a similar feature is observed frequently in the corpus callosum, basal ganglia, hippocampus, lateral geniculate body, thalamus, cerebellum, and cerebral subcortex, and it is observed infrequently in the midbrain, internal capsule, and medulla oblongata710). Experimental data suggest that the location of brain lesions depends on the susceptibility to osmotic stress and oligodendrocytes are most susceptible to osmotic stress10). Use of conventional MRI sequences with T1- or T2-weighted images often delays the diagnosis12). Currently, it appears that DW imaging with ADC mapping yields higher specificity for ODS lesions with early signal changes after the onset of neurologic symptoms and it may be helpful for early diagnosis of ODS1314). In an analysis of the reported literature data of ODS from 24 hypernatremic patients, the predilection site was more common in EPM(n=10) than in combined CPM/EPM(n=9) or isolated CPM(n=5)7). In our case, lesions were located in both pontine and extrapontine sites. However, the pontine lesion showed an unusual pattern involving the lateral part of the pons with sparing of both central and peripheral fibers. In our opinion, like differences in the predilection site of CPM and EPM, regional differences in susceptibility to osmotic stress within the pons might be responsible for the unusual pattern.
Reported clinical features of CPM included tetraparesis, pseudobulbar palsy, early depression of consciousness level, seizure, cerebellar ataxia, and other brainstem features. Our patient had tetraparesis and prominent flaccidity, which have especially been described soon after the onset of ODS.
The principles of the treatment of hypernatremia are the correction of the underlying causes and the prevailing hypertonicity. Especially, the rate of correction of hypernatremia is important because it is related to fetal brain edema. The standard recommendation for the correction rate is that because hypernatremia develops over a period of hours, rapid correction that reduces the serum sodium concentration by 1mEq/L/hour improves the prognosis. For longer or unknown duration, reduction in the serum sodium concentration at a maximal rate of 0.5mEq/L/hour or a fall in the serum sodium concentration of 10mEq/L/day is recommended to prevent cerebral edema9). In our case, the correction rate of hypernatremia was significantly greater than the recommended correction rate (15mEq/L over the first 24 hours). Fortunately, the patient did not develop brain edema and convulsions.
Recently, there have been reports suggesting that corticosteroids may improve the outcome of hypernatremic demyelination. Dexamethasone had a protective role against osmotic induced demyelination in rats10) and five patients who received corticosteroids had better outcomes11). Corticosteroids protect the brain blood barrier and play a critical role in protecting against demyelination12). However, we used dexamethasone 5mg and hydrocortisone 100mg/day considering her history of steroid use for protection from adrenal insufficiency. We used steroids solely to prevent adrenal insufficiency; hence, we do not know exactly what are the effects of corticosteroid therapy for prevention of ODS.
In summary, this is the first report of a successful treatment of hypernatremia caused by iatrogenic salt intake with corticosteroids, and it confirms the importance of adequate fluid and corticosteroid supplementation in severe hypernatremia.
References
1. Samuels MA, Seifter JL. Encephalopathies caused by electrolyte disorders. Semin Neurol. 2011; 31:135–138. PMID: 21590618.

2. Naik KR, Saroja AO. Seasonal postpartum hypernatremic encephalopathy with osmotic extrapontine myelinolysis and rhabdomyolysis. J Neurol Sci. 2010; 291:5–11. PMID: 20129624.

3. Arieff AI. Central nervous system manifestations of disordered sodium metabolism. Clin Endocrinol Metab. 1984; 13:269–294. PMID: 6488574.

5. An JY, Park SK, Han SR, Song IU. Central pontine and extrapontine myelinolysis that developed during alcohol withdrawal, without hyponatremia, in a chronic alcoholic. Intern Med. 2010; 49:615–618. PMID: 20228603.

6. Ismail FY, Szollics A, Szolics M, Nagelkerke N, Ljubisavljevic M. Clinical semiology and neuroradiologic correlates of acute hypernatremic osmotic challenge in adults: A literature review. Am J Neuroradiol. 2013; 34:2225–2232. PMID: 23413245.

7. Machino T, Yoshizawa T. Brain shrinkage due to acute hypernatremia. Neurology. 2006; 67:880. PMID: 16966557.

8. Ayus JC, Armstrong DL, Arieff AI. Effects of hypernatraemia in the central nervous system and its therapy in rats and rabbits. J Physiol. 1996; 492:243–255. PMID: 8730599.

10. Sugimura Y, Murase T, Takefuji S, Hayasaka S, Takagishi Y, Oiso Y, et al. Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol. 2005; 192:178–183. PMID: 15698632.

11. Sakamoto E, Hagiwara D, Morishita Y, Tsukiyama K, Kondo K, Yamamoto M. Complete recovery of central pontine myelinolysis by high dose pulse therapy with methylprednisolone. Nihon Naika Gakkai Zasshi. 2007; 96:2291–2293. PMID: 18044169.

12. Adler S, Verbalis JG, Meyers S, Simplaceanu E, Williams DS. Changes in cerebral blood flow and distribution associated with acute increases in plasma sodium and osmolality of chronic hyponatremic rats. Exp Neurol. 2000; 163:63–71. PMID: 10785445.

13. Cramer SC, Stegbauer KC, Schneider A, Mukai J, Maravilla KR. Decreased diffusion in central pontine myelinolysis. Am J Neuroradiol. 2001; 22:1476–1479. PMID: 11559493.
14. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. Am J Neuroradiol. 2004; 25:210–213. PMID: 14970019.
Fig. 1
Clinical course of the patient. The upper graph shows the changes in serum sodium and osmolality. The lower part shows the serum creatine phosphokinase level. Brain magnetic resonance imaging was performed on day 6.
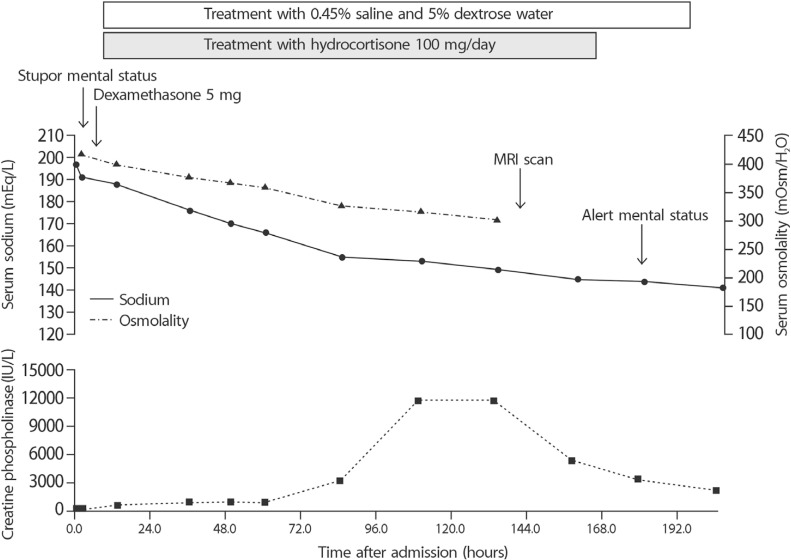
Fig. 2
Initial MRI shows multifocal diffusion restriction in bilateral pons and bilateral thalami on DWI (B and E) and low signal intensity lesion in the same portion on ADC maps (C and F). There were very subtle high signal intensity lesions in bilateral central pons on the FLAIR images (A and D). ADC: apparent diffusion coefficient, DWI: diffusion-weighted image, FLAIR: fluid-attenuated inversion recovery, MRI: magnetic resonance image.
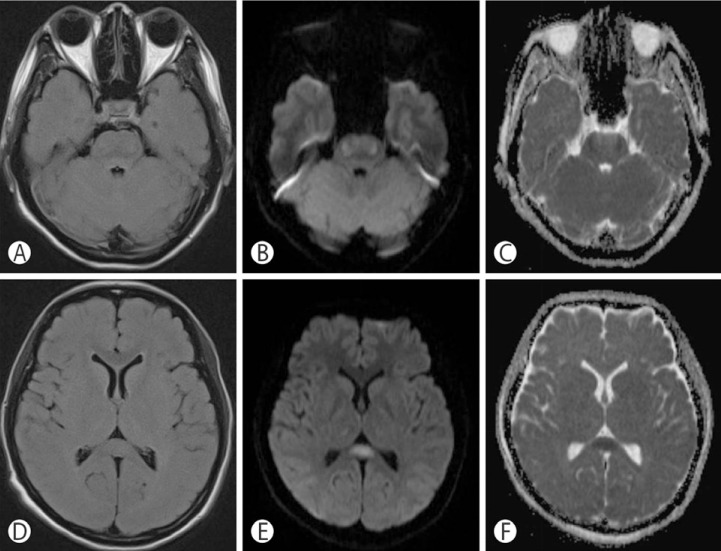
Fig. 3
Follow-up MRI performed on hospital day 30 shows symmetric high signal intensity changes in the lateral pons and bilateral thalami on coronal T2-weighted image (A). Previously noted diffusion restriction in bilateral pons and bilateral thalami had resolved on ADC maps (D and G) and diffusion restriction in bilateral central mid-pons, upper pons, and bilateral thalami had disappeared on DWI (B, C, and F).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download