This article has been
cited by other articles in ScienceCentral.
Abstract
Escape from the renal actions of vasopressin is said to occur in rats with chronic hyponatremia. Our objective was to provide specific evidence to test this hypothesis. Hence the osmolality in the excised renal papilla and in simultaneously voided urine (UOsm) was measured in rats with and without hyponatremia. To induce hyponatremia, rats were fed low-electrolyte chow for 6 days. In the first 3 days, water was provided ad lib. On days 4 to 6, a long acting vasopressin preparation (dDAVP) was given every 8 hours to induce water retention. The hyponatremic rats drank 21 mL 5% sucrose on day 4 and 6 mL on day 5. On the morning of day 6, these rats were given 10 mL of 5% glucose in water (D5W) by the intraperitoneal route at 09:00 hour and at 11:00 hour. Analyses were performed in blood, urine, and the excised renal papilla at 13:00 hour on day 6. The concentration of Na+ in plasma (PNa) in rats without intraperitoneal D5W was 140±1 mEq/L (n=7) whereas it was 112±3 mEq/L in the hyponatremic group (n=12). The hyponatremic rats had a higher osmolality in the excised papillary (1,915±117 mOsm/kg H2O) than the UOsm (1,528±176 mOsm/kg H2O, P<0.05). One explanation for this difference is that the rats escaped from the renal action of vasopressin. Nevertheless, based on a quantitative analysis, other possibilities will be considered.
Keywords: vasopressins, aquaporins, basal water permeability, concentration of the urine
Introduction
It is widely held that the kidneys 'adapt' to the presence of chronic hyponatremia by escaping from the actions of vasopressin
1-
8). This implies that many fewer aquaporin 2 water channels (AQP2) were inserted in the luminal membranes of the late distal nephron in presence of actions of vasopressin. If true, the late distal nephron should become sufficiently impermeable to water so that the osmolality in the papillary interstitial compartment is higher than in the urine (U
Osm). This osmolality difference must be sustained in steady state. The implied advantage of this escape from the actions of vasopressin is to lessen the degree of chronic hyponatremia. Thus, a large sustained fall in the concentration of sodium (Na
+) in plasma (P
Na) should induce a signal to the kidneys to excrete some of the extra water that was retained to lower the P
Na1-
8). Hence, one would not expect to see this difference in osmolality if hyponatremia were not present.
Three indirect lines of investigation provide support for the hypothesis of escape. First, the U
Osm should be significantly lower during sustained hyponatremia in rats that had persistent actions of vasopressin (not tested). Second, the number of AQP2 in the lumen of the late distal nephron was dramatically reduced in these rats
1,
6). Third, there were fewer V
2 receptors for vasopressin in the late distal nephron. Hence these receptors were down-regulated in the rats with escape from the renal actions of vasopressin
9).
There are some factors that call into question, the physiologic importance of escape from the actions of vasopressin. First, the U
Osm in the rats that underwent this escape is quite high, often more than 5-fold higher than the osmolality in plasma (P
Osm) in hyponatremic rats. Second, if the difference in the osmolality between the papillary interstitial compartment and the urine is large, this will imply that there is an enormous driving force for water reabsorption at a time when ~90% of the water delivered to the late distal nephron was reabsorbed (see the Discussion for more details concerning this statement). Third, it is not clear how many AQP2 channels are needed to allow water to diffuse to osmotic equilibration. Moreover, in addition to the number of AQP2 and the osmotic driving force for water movement through these channels, the contact time for water diffusion and turbulence to stir the luminal compartment may influence the absolute volume of water that will diffuse through AQP2. Fourth, although V
2 receptors for vasopressin are down-regulated in the rats with escape from the renal actions of vasopressin
9), this does not constitute proof that there were insufficient AQP2 water channels and thereby, cause escape from the renal actions of vasopressin.
Accordingly, our objective was to test the escape hypothesis directly by determining the magnitude of the difference in osmolality between the excised renal papilla and the urine in rats with a range of PNa values in the hyponatremic range. Results to be reported indicate that only in the rats with the mean PNa of 112 mEq/L was the papillary osmolality significantly higher that the UOsm. Nevertheless, this difference in osmolality was only ~20% of the osmolality in the excised papilla. Although this difference in osmolality was relatively small, it would still exert a huge driving force for the movement of water in the renal papilla. Notwithstanding, because a severe degree of hyponatremia was required to elicit this osmotic difference and that there was other evidence to indicate that these very hyponatremic animals may be catabolic, this raises doubts about the physiologic importance of escape from the actions of vasopressin.
Methods
1. Animals
Adult male Wister rats (weight 300-400 g) were used in this study; they were cared for in accordance with the principles and guidelines of the Canadian Council on Animal Care. The Animal Care Committee of St. Michael's Hospital approved the study protocol.
2. Preliminary studies
These were designed to develop a simple model where rats would have a lower PNa owing to the retention of ingested water. Accordingly, 2 groups of rats were studied, the first (n=7) was fed regular rat chow and the second (n=9) was fed a low-electrolyte diet for 6 days (Na+ 1 mEq/kg, K+ 0 mEq/kg, Cl- 0 mEq/kg, ICN Pharmaceuticals, Montreal, Canada). Hyponatremia was only produced in rats consuming the low electrolyte diet. Hence all studies to be reported are with rats on the low electrolyte diet.
3. Experimental studies to evaluate the composition of the renal papilla
Studies were carried out in 19 rats fed the low-electrolyte diet for 6 days. To ensure persistent V2 actions of vasopressin, all rats were given 2 µg of desmopressin acetate (dDAVP, Ferring Co., Ontario, Canada) by the intraperitoneal route every 8 hours on days 4 and 5, and on the morning of day 6. The drinking solution was changed to 5% sucrose to encourage its consumption on days 4 and 5. The volume consumed was 15 mL/day in the control group and 21 mL on day 4 and 6 mL on day 5 in the hyponatremia group. Since sugar water consumption declined on day 5, the experimental group of rats was given 10 mL of 5% glucose in water by the intraperitoneal route at 09:00 hour and at 11:00 hour on the morning of day 6. Analyses were performed in blood and urine at 13:00 hour on day 6.
The kidneys were removed for excision of the renal papilla as previously described
10). The kidney was sliced obliquely along its longitudinal axis with a sharp knife to expose the intact papilla. The papillary tip was blotted, excised, and transferred immediately to a pre-weighed plastic vial and sealed. After the vial plus the papilla was weighed, 1 mL of the solution used for flame photometric measurement of Na
+ and K
+ was added to this vial and the tissue was homogenized. This fluid was analyzed for urea, Na
+, K
+, and NH
4+. To determine the water content of the renal papilla, the papilla of the second kidney was handled as described above; it was desiccated and the vial was weighed again. The difference in weight was used to reflect its water content
10).
4. Analytical techniques
Na
+ and K
+ in plasma and urine were determined by flame photometry (Radiometer, FLM-3, London, ON, Canada). Gas analysis in blood and urine was performed at 37℃ with a digital pH/blood gas analyzer (Corning 178 blood pH analyzer). Osmolality was measured by freezing point depression (Advanced Instruments Inc, Needham Heights, MA, USA). NH
4+, urea, and creatinine were measured as previously described
11,
12).
5. Statistical analysis
Results are reported as mean±SEM. Statistical analysis was performed on the group mean values for the parameters in blood and in the urine using an unpaired Student t-test. For comparisons of osmolalities in the excised papilla and the UOsm, the differences in osmolality were compared using a paired Student t-test. A P value that was less than 0.05 was considered to be statistically significant.
Results
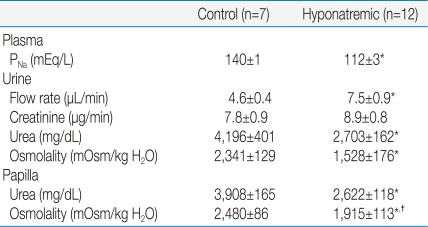
The P
Na was in the normal range (140±1 mEq/L, n=7) in the control group (
Table 1); these rats had a U
Osm of 2,341±129 mOsm/kg H
2O. The osmolality in their excised papilla was not significantly different from their U
Osm (2,480±86 mOsm/kg H
2O). In the 12 rats with a P
Na of 112±3 mEq/L, the U
Osm was 1,528±176 mOsm/kg H
2O, which was significantly lower than the osmolality in the excised papilla (1,915±113 mOsm/kg H
2O,
P<0.05) by paired analysis.
The control group consumed 15 g of the low electrolyte chow on both days 4 and 5, whereas rats with the low PNa consumed less chow on days 4 and 5 (4 g/day). The rats with a PNa of 112 mEq/L weighed 402±6 g on the morning of day 4. Despite a 20% decline in their PNa on the morning of day 6, which implies a large gain of water in their cells, these rats weighed only 389±7 g. Hence they had lost a considerable amount of lean body mass (i.e., they were catabolic).
Discussion
The major new observation is that rats with a P
Na of 112 mEq/L had a statistically significantly higher osmolality in their excised renal papilla (1,915±113 mOsm/kg H
2O, higher) than their simultaneously voided urine (1,528±176 mOsm/kg H
2O,
P<0.05 by paired analysis). These results are consistent with escape from the renal actions of vasopressin. Nevertheless, since there were no significant differences in the concentrations of urea in the urine and in the papillary interstitial compartment, there was no escape from the ability of vasopressin to insert urea transporters in the luminal membranes of the inner medullary collecting duct (
Table 1).
Since there was a large rise in the osmolality as fluid descended through the medullary collecting duct (i.e., from a POsm of 224 mOsm/kg H2O to ~1,500 mOsm/kg H2O), other possible explanations for the lower UOsm will be considered. We shall begin with quantitative analyses to place this observation in physiologic perspective, discuss possible links between hyponatremia and a lower medullary interstitial osmolality, and consider alternative explanations for the somewhat lower UOsm in the urine of these very hyponatremic rats.
1. Quantitative analyses
How large is the driving force to reabsorb water in the inner medullary collecting duct? An osmolality difference between the papillary interstitial compartment and the urine of 300 mOsm/kg H
2O, which is similar to the measured osmotic difference in our experiments (
Table 1), exerts an enormous force to reabsorb water when AQP2 are present. In numeric terms, this difference in osmolality must be multiplied by 19.3 mm Hg, the osmotic driving force when there is a difference of 1 mOsm/kg H
2O. Hence, the osmotic driving force is 5,790 mm Hg. Comparing this number to the mean arterial blood pressure in the rat (~100 mm Hg), this osmotic driving force is equivalent to the mean pressure exerted by 58 beating hearts.
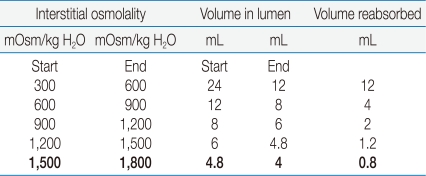
What is the quantitative significance of a 300 mOsm/kg H
2O lower U
Osm on the volume of water reabsorbed in the medullary collecting duct? The driving force to reabsorb water is virtually identical at each horizontal plane in the renal medulla if the osmolality were to rise by 300 mOsm/kg H
2O (with a small correction needed for changes in ionic strength). Notwithstanding, the volume of water reabsorbed in each successive 300 mOsm/kg H
2O rise in osmolality declines markedly (
Table 2). Thus, if the U
Osm was 300 mOsm/kg H
2O lower than the osmolality in the excised papilla in the rats with a P
Na of 112 mEq/L, the escape phenomenon would result in the excretion of only 0.8 mL more water per day (see the bottom line in
Table 2). This is a very small excretion of extra water, which raises questions about the physiologic importance of vasopressin escape.
2. Evaluation of the evidence for the diminished permeability to water in the inner medullary collecting duct
Defects have been identified in vasopressin binding to its V2 receptor, the generation of second messengers, and response elements that lead to a decreased permeability of the inner medullary collecting duct to water.
1) Diminished total binding of a V2 selective receptor antagonist to the inner medullary collecting duct
Although this has been demonstrated
9), there was still appreciable binding of this ligand to the V
2 receptor in the inner medullary collecting duct in these rats. Hence, these data do not constitute irrefutable evidence concerning the validity of this hypothesis.
2) Downstream events to the V2 receptor for vasopressin
There was an impressive reduction in AQP2 in kidney homogenates, a similar degree of reduction in osmotically driven water permeability in the inner medullary collecting duct, and a reduction, although to a lesser degree, in the generation of cyclic AMP following exposure of this nephron segment to dDAVP in this in vitro setting
2). While these data seem to support to the hypothesis of vasopressin escape, it is not clear whether the decrease in AQP2 in the inner medullary collecting duct is sufficient to limit a quantitatively important volume of water reabsorption in this nephron segment in vivo.
3) Escape from urea transporter insertion when vasopressin acts
In addition to its effect to cause the insertion of AQP2 in the luminal membrane of principal cells in the inner medullary collecting duct, vasopressin also causes the insertion of urea transporters in the luminal membrane of cells in this nephron segment. For example, if there was reduced reabsorption of urea in the inner medullary collecting ducts, the concentration of urea could fall in the papillary interstitial compartment. Since urea constitutes ~50% of the urine osmoles the result would be a decreased U
Osm and a larger urine flow rate owing to both the lower papillary interstitial osmolality and a larger number of effective osmoles in the urine (urea has become an effective urine osmole)
10,
13). In the present study, this aspect of escape was tested by measuring the concentrations of urea in the papillary interstitial compartment and in the urine. As shown in
Table 1, there was no significant difference in the concentration of urea in the papilla and in the urine. Hence, there is no evidence to support an escape from this action of vasopressin. Thus, if escape is a valid hypothesis, the mechanism must affect the insertion of AQP2, but not the insertion of urea transporters in the inner medullary collecting duct.
4) Lower medullary interstitial osmolality without water addition from the inner medullary collecting duct
The hallmark of vasopressin escape is a decrease in the U
Osm to ~2,000 mOsm/kg H
2O
1). On the other hand, since the U
Osm is this high, clearly there was enough permeability to water to permit the osmolality in luminal fluid in the beginning of the medullary collecting duct to rise ~7-fold in the hyponatremic rats, which means that 86% of delivered water was reabsorbed via AQP2 in the medullary collecting duct in rats with vasopressin escape. In addition, if there was very little water reabsorbed in the inner medullary collecting duct, this would cause a smaller fall in medullary interstitial osmolality because there would be a smaller degree of medullary washout.
A lesion resembling escape could be present if there was an appreciable rise in the rate of medullary blood flow
5). If this were indeed responsible for the lower papillary interstitial osmolality, calling the process escape from the renal actions of vasopressin in the inner medullary collecting duct might be the wrong conclusion to draw in mechanistic terms.
5) The physiologic importance of vasopressin escape
Verbalis stated that escape from the renal actions of vasopressin allows survival of the organism by allowing free water excretion despite inappropriate secretion of vasopressin, thus, effectively antagonizing the effects of one of the most powerful hormones involved in body fluid homeostasis
8). Nevertheless, the model of an infusion of dDAVP with overriding the thirst mechanism does not represent the physiology of the water control system in our opinion. Said another way, although it represents an experimental model of considerable interest, it is difficult to see how this would provide a survival advantage in Paleolithic times. Moreover, at times, patients with hyponatremia may have a large deficit of Na
+ and thereby, a very contracted extracellular fluid (ECF) volume. Hence, if escape were to be triggered by a low P
Na in this setting, there may be a threat for hemodynamic reasons if an appreciable amount of water was excreted. Overall, we agree with the conclusion of Verbalis
8) that this hypothesis must remain a story in evolution.
3. Other features associated with vasopressin escape and hyponatremia
1) Why may the medullary interstitial osmolality fall in rats with chronic hyponatremia?
There are two major reasons for a lower medullary interstitial osmolality in this setting.
(1) A lower arterial P
Na can influence the maximum U
Osm because if the interstitial osmolality can be ~10-fold higher than the P
Osm, this osmolality will be ~3,000 mOsm/kg H
2O in rats with a normal P
Na (10×the P
Osm of 300 mOsm/kg H
2O). On the other hand, if the same 10-fold rise occurred when the P
Na was 112 mEq/L (the P
Na where we observed data compatible with the phenomenon of vasopressin escape,
Table 1), the maximum U
Osm would be 2,240 mOsm/kg H
2O (10×224 mOsm/kg H
2O).
(2) When more water is added to the medullary interstitial compartment, its osmolality might fall. In fact, the volume of water reabsorbed in the major water-permeable nephron segment that traverses the outer medulla (i.e., the medullary collecting duct) should be higher if a larger volume of filtrate is delivered to the medullary collecting duct and vasopressin acts. This delivery volume will be higher if more osmoles arrive at this nephron segment per unit time and/or if there is a lower P
Osm (equation 1)
14). Since the P
Osm was ~20% lower in the rats with a P
Na of 112 mEq/L, the delivery of fluid to the medullary collecting duct should be higher and thus, more water should be added to this medullary interstitial compartment in hyponatremic rats. Therefore, a lower papillary interstitial osmolality can occur independent of escape from the actions of vasopressin in rats with hyponatremia.
Volume delivered to the medullary collecting ducts = # osmoles delivered/POsm (1)
2) Does a maximum UOsm ~1,500 mOsm/kg H2O during hyponatremia constitute proof for vasopressin escape?
While this lower than expected U
Osm could be due to escape form the renal actions of vasopressin, there are factors other than the number of luminal AQP2 that may reduce water reabsorption in the inner medullary collecting duct. These other factors include the contact time available for facilitated diffusion of water and the creation of turbulent flow in the lumen of the inner medullary collecting duct, which 'stirs' this fluid to aid the diffusion process. These two factors are influenced primarily by renal pelvic contraction and retrograde flow from the renal pelvis into the inner medullary collecting duct
15). It is not known what factors may influence the strength and/or frequency of contraction of the renal pelvis, but it is possible that this reduction could occur in rats with cachexia. In fact, our hyponatremic rats did not gain weight despite the fact that they should have a larger intracellular fluid (ICF) volume. In quantitative terms, the expected weight gain in a 390 g rat can be calculated from an estimate of total body water (67% of 390 g=260 mL), of which ~2/3 of this volume is in the ICF (~175 mL). With a 20% decline in the P
Na, the ICF volume expansion should be 35 mL (or a weight gain of 35 g). Thus they may have become cachectic because they ingested less food or due to the hyponatremia per se.
3) What other mechanisms can produce results that resemble the escape from actions of vasopressin?
Three obvious factors will be considered:
(1) Down regulation of the V2 receptors for vasopressin
The number of vasopressin receptors, like many other hormone receptor levels, decline when the concentration of their ligand in plasma rises (i.e., down regulation)
16). Nevertheless, down regulation does not necessarily lower the number of hormone-hormone receptor complexes, as their number is a function of the rise in the concentration of the ligand, its affinity for the receptor, and the percentage decline in the number of receptors. Of note, all three groups of rats received the same dose of dDAVP. Moreover, if the mechanism for escape were simply a response to high vasopressin levels, water deprived subjects in the desert could develop a larger deficit of water, which would pose a threat to survival in this setting.
(2) Difficulty in knowing if a constant volume of water is absorbed per AQP2 water channel
There are two problems in this context. First the driving force to cause the movement of water (osmotic pressure difference between the medullary interstitial compartment and the luminal fluid) influences the volume of water traversing an open water channel. Second, the contact time whereby luminal fluid is present in nephron segments with AQP2 is another variable. Hence one cannot directly correlate the volume of filtrate reabsorbed with the number of AQP2 water channels seen on morphologic studies.
4. Concluding remarks
While data compatible with vasopressin escape were obtained in our study, they were observed in rats with a severe degree of hyponatremia (
Table 1). In quantitative terms, the difference in osmolality observed would have a very small effect on the excretion of water because these rats could achieve a U
Osm of ~1,500 mOsm/kg H
2O (
Table 2). It is important to recognize that these rats likely had an appreciable loss of lean body mass because they failed to gain weight, yet they had close to a 20% decline in their P
Na - the latter implies that there was a 20% expansion of their ICF volume, which is equivalent to a 35 g weight gain. Since there was no weight gain in these rats, they had hyponatremia plus a catabolic state. Hence, caution is needed when interpreting the results of proteomics in this population
17), as one cannot be sure whether the changes reflect hyponatremia and/or catabolism. Finally, there is another possible interpretation of the difference in osmolality between the urine and the papillary interstitial compartment, reduced renal pelvic contraction and thereby, retrograde flux in the inner medullary collecting duct, as this could have an important effect on facilitated diffusion of water that is restricted to the this latter nephron segment.
Acknowledgements
The authors wish to acknowledge the excellent technical assistance of Stella Tang and SY Lee for her most valuable secretarial contributions. The authors are extremely grateful to the Squires Club, and the Divisions of Research and Nephrology of St Michaels Hospital and the CIHR for financial support to carry out this research.
References
1. Ecelbarger CA, Nielsen S, Olson BR, et al. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest. 1997; 99:1852–1863. PMID:
9109429.

2. Ecelbarger CA, Chou CL, Lee AJ, DiGiovanni SR, Verbalis JG, Knepper MA. Escape from vasopressin-induced antidiuresis: role of vasopressin resistance of the collecting duct. Am J Physiol. 1998; 274:F1161–F1166. PMID:
9841509.
3. Ecelbarger CA, Knepper MA, Verbalis JG. Increased abundance of distal sodium transporters in rat kidney during vasopressin escape. J Am Soc Nephrol. 2001; 12:207–217. PMID:
11158210.

4. Hoorn EJ. Water and salt: from renal mechanisms to clinical disorders [dissertation]. 2007. Rotterdam: Erasmus Universiteit Rotterdam.
5. Murase T, Tian Y, Fang XY, Verbalis JG. Synergistic effects of nitric oxide and prostaglandins on renal escape from vasopressin-induced antidiuresis. Am J Physiol Regul Integr Comp Physiol. 2003; 284:R354–R362. PMID:
12388460.
6. Murase T, Ecelbarger CA, Baker EA, Tian Y, Knepper MA, Verbalis JG. Kidney aquaporin-2 expression during escape from antidiuresis is not related to plasma or tissue osmolality. J Am Soc Nephrol. 1999; 10:2067–2075. PMID:
10505682.

7. Song J, Hu X, Khan O, Tian Y, Verbalis JG, Ecelbarger CA. Increased blood pressure, aldosterone activity, and regional differences in renal ENaC protein during vasopressin escape. Am J Physiol Renal Physiol. 2004; 287:F1076–F1083. PMID:
15226153.

8. Verbalis JG. Escape from antidiuresis: a good story. Kidney Int. 2001; 60:1608–1610. PMID:
11576381.

9. Tian Y, Sandberg K, Murase T, Baker EA, Speth RC, Verbalis JG. Vasopressin V2 receptor binding is down-regulated during renal escape from vasopressin-induced antidiuresis. Endocrinology. 2000; 141:307–314. PMID:
10614652.
10. Gowrishankar M, Lenga I, Cheung RY, Cheema-Dhadli S, Halperin ML. Minimum urine flow rate during water deprivation: importance of the permeability of urea in the inner medulla. Kidney Int. 1998; 53:159–166. PMID:
9453013.

11. Cheema-Dhadli S, Halperin ML. Relative rates of appearance of nitrogen and sulphur: implications for postprandial synthesis of proteins. Can J Physiol Pharmacol. 1993; 71:120–127. PMID:
8319135.

12. Halperin ML, Vinay P, Gougoux A, Pichette C, Jungas RL. Regulation of the maximum rate of renal ammoniagenesis in the acidotic dog. Am J Physiol. 1985; 248:F607–F615. PMID:
3985167.

13. Gamble J, McKhann C, Butler A, Tuthill E. An economy of water in renal function referable to urea. Am J Physiol. 1934; 109:139–154.

14. Steele A, deVeber H, Quaggin SE, Scheich A, Ethier J, Halperin ML. What is responsible for the diurnal variation in potassium excretion? Am J Physiol. 1994; 267:R554–R560. PMID:
8067468.

15. Schmidt-Nielsen B, Churchill M, Reinking LN. Occurrence of renal pelvic refluxes during rising urine flow rate in rats and hamsters. Kidney Int. 1980; 18:419–431. PMID:
7230608.

16. Robinson AG, Roberts MM, Evron WA, Verbalis JG, Sherman TG. Hyponatremia in rats induces downregulation of vasopressin synthesis. J Clin Invest. 1990; 86:1023–1029. PMID:
2211999.

17. Hoorn EJ, Hoffert JD, Knepper MA. Combined proteomics and pathways analysis of collecting duct reveals a protein regulatory network activated in vasopressin escape. J Am Soc Nephrol. 2005; 16:2852–2863. PMID:
16079266.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download