TO THE EDITOR: Adult T-cell leukemia/lymphoma (ATLL) is a malignancy of mature CD4+ T-cells, and is caused by the human T-cell lymphotropic virus type 1 (HTLV-1), which has infected 20 million people throughout the world, primarily in Africa, the Caribbean, and South America. We report on a typical case of a 50-year-old man with a marked leukocytosis, multiple organ involvement, and an aggressive outcome. To the best of our knowledge, this is the eighth case report of ATLL in Korea.
A 50-year-old man was referred to the Department of Hematology with leukocytosis, following observation of multiple cervical lymphadenopathy by a general practitioner. He had no history of traveling abroad, and had been 12 months. Multiple erythematous patches were observed on his back, which were considered to be anti-tuberculous medication drug eruptions (Fig. 1A). His initial laboratory tests revealed the following: WBC 60,800/µL, Hb 10.2 g/dL, Hct 31.6%, and platelets 138,000/µL. Computed tomographic (CT) scans revealed the enlargement of multiple cervical lymph nodes (Fig. 1B) with marked hepatosplenomegaly (Fig. 1C), and one lymph node was submitted for pathological diagnosis.
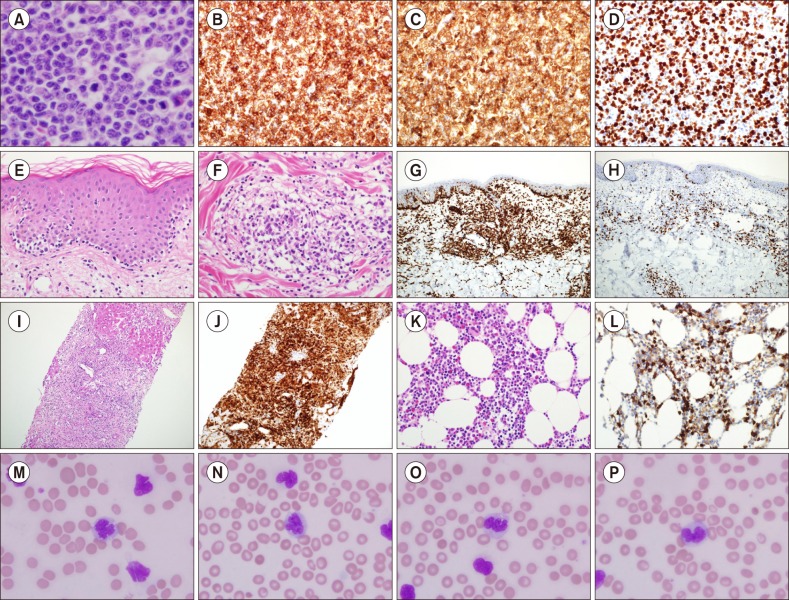
Microscopic examination revealed a totally effaced and enlarged lymph node that consisted of medium-sized atypical lymphoid cells, which had marked irregular nuclear contours with prominent nucleoli (Fig. 2A). No necrotic foci were detected. The immunohistochemical studies showed that the atypical cells were positive for CD3, CD4, and a high Ki-67 proliferation index (Fig. 2B–D), whereas CD20, CD79a, CD5, CD8, CD56, CD30 and EBV-encoded ribonucleic acids (EBER) determined through in situ hybridization were negative. A biopsy was performed to evaluate the skin lesions. Biopsy results revealed massive atypical lymphoid cell infiltration into the superficial perivascular spaces with epidermotropism resembling Mycosis fungoides (Fig. 2E, F), which were also positive for CD3, CD4 (Fig. 2G), and a high Ki-67 labelling index (Fig. 2H). A liver biopsy was performed and the results showed that the hepatocytes had been markedly destroyed by atypical lymphoid cells. Immunohistochemical stain results were identical with his lymph node and skin lesions (Fig. 2I, J). During the evaluation, a bone marrow aspiration and biopsy were performed. The bone marrow showed 50% of cellularity with mostly CD3 and CD4 positive atypical lymphoid cells (Fig. 2K, L). His leukocytosis became gradually apparent and his WBC was at 103,100/µL, comprising 73% lymphocytes fraction and 5% atypical lymphocytes, and 3,282 IU/L of lactate dehydrogenase (LDH) and elevated serum calcium (10.8 mg/dL) levels were recorded. The peripheral blood smear identified large flower cells with convoluted and lobulobulated nuclei (Fig. 2M–P). Eventually, anti-HTLV-1 Ab was detected in his blood and the diagnosis of ATLL was confirmed. He received a second cycle of hyper-CVAD chemotherapy; however, pneumonia developed as a complication and he died 132 days after diagnosis.
ATLL was first described in 1977 as a distinct progressive T-cell leukemia of peculiar morphology, highlighted by so-called ‘flower cells’ with a suspected viral etiology, due to clustering of the disease in the southwestern region of Japan [1]. Subsequently, a novel RNA retrovirus, namely HTLV-1, was isolated from a cell line established from leukemic cells of an ATLL patient, and the finding of a clear association with ATLL was proven as a human carcinogenic pathogen [2]. Prototypical ATLL cells have a mature helper T-cell phenotype (CD3+, CD4+, CD8−), and recent studies have suggested that the cells of some ATLL patients may be the equivalent of regulatory T-cells because of the high frequency of expression of CD25/CCR4, and approximately half that of FoxP3 [3].
Based on the natural history, clinical characteristics, and prognosis, ATLL is classified into five clinical types, as follows: smoldering, chronic, primary cutaneous tumoral (PCT), lymphoma, and acute. The smoldering type is subdivided into leukemic and non-leukemic, and the chronic type is subdivided into favorable and unfavorable. The acute, lymphoma, unfavorable chronic, and PCT types are considered aggressive, while the favorable chronic and non-leukemic smoldering types have a better prognosis [4].
ATLL cells, the so-called ‘flower cells’, are usually detected easily in the blood of affected individuals, except in the smoldering type [5], and are seen as medium and/or large lymphocytes with multi-lobulated nuclei, dense chromatin, and absent or small nucleoli. These cells are considered pathognomonic of ATLL and enable diagnosis alone. ATLL cells in the skin and lymph node can vary in size from small to large, and from pleomorphic to anaplastic, and may even appear as Hodgkin-like cells with no specific histological pattern of involvement. Therefore, distinguishing ATLL from Sezary syndrome, peripheral T-cell lymphoma, and Hodgkin lymphoma can be difficult without detection of the HTLV-1 serotype/genotype [6].
Hypercalcemia is the most distinctive laboratory abnormality in ATLL, as compared to other lymphoid malignancies, and is observed in 31% of patients [5]. The major prognostic indicators for ATLL, identified among 854 patients in Japan with ATLL using multivariate analysis, are as follows: advanced performance status, high LDH level, 40 years of age or over, involving more than three lesions, and hypercalcemia [7]. The best results for the aggressive clinical forms (acute, lymphoma, and unfavorable chronic) were obtained with the VCAP-AMP-VECP regimen (vincristine, cyclophosphamide, doxorubicin, prednisone- doxorubicin, ranimustine, and prednisone- vindesine, etoposide, carboplatin, and prednisone). However, due to the high toxicity of this regimen, particularly in patients over 70 years of age, the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimens are preferred. Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone - methotrexate, and cytarabine) is also acceptable as an alternative regimen [8].
The clinicopathologic characteristics of ATLL cases experienced in Korea are summarized in Table 1. The patients ranged in age from 28 to 64 years, with a mean age of 50.3 years [9101112131415]. All the cases presented with skin lesions (100%), and leukocytosis and bone marrow involvement with atypical lymphocytes in the peripheral blood were proven in seven cases (87.5%) [91011121415]. Skin involvement due to ATLL has been reported in approximately 60% of cases and is frequently found in the smoldering and chronic forms [3]. Interestingly, all the Korean cases of ATLL showed skin lesions, mostly in the acute clinical type.
In summary, we reported a case of ATLL in a 50-year-old Korean man who had never travelled abroad, and with no history of transfusion. He presented with the typical symptoms of ATLL including leukocytosis, hypercalcemia, elevation of LDH, lymphadenopathy, skin, liver, and bone marrow involvement, and an aggressive clinical course, which has been rarely observed in Korea. Because the histopathological patterns of ATLL vary and mimic different types of T-cell lymphomas, such as peripheral T-cell lymphoma, NOS, Mycosis fungoides or, less often, anaplastic large cell lymphoma, awareness of an HTLV-1 infection and ATLL may lower the possibility of misdiagnosis in non-endemic areas.
References
1. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977; 50:481–492. PMID: 301762.

2. Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982; 79:2031–2035. PMID: 6979048.

3. Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003; 9(10 Pt 1):3625–3634. PMID: 14506150.
4. Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009; 27:453–459. PMID: 19064971.

5. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991; 79:428–437. PMID: 1751370.
6. Tsukasaki K, Tobinai K. Biology and treatment of HTLV-1 associated T-cell lymphomas. Best Pract Res Clin Haematol. 2013; 26:3–14. PMID: 23768636.

7. Major prognostic. a cooperative study. Lymphoma Study Group (1984–1987). Leuk Res. 1991; 15:81–90. PMID: 2016910.
8. Di Venuti G, Nawgiri R, Foss F. Denileukin diftitox and hyper-CVAD in the treatment of human T-cell lymphotropic virus 1-associated acute T-cell leukemia/lymphoma. Clin Lymphoma. 2003; 4:176–178. PMID: 14715100.
9. Lee MH, Kim BK, Lee HB, et al. Adult T-cell leukemia - The first case in the Republic of Korea. J Korean Med Assoc. 1987; 30:1146–1152.
10. Kim JB, Kim KJ, Kim SY, et al. A case of adult T-cell leukemia/lymphoma in Korea. Korean J Med. 1994; 47:699–704.
11. Lee SS, Hong SI, Lee DS, Kang YK, Kim CW, Jang JJ. Adult T-cell leukemia/lymphoma in a Korean: a case report. J Korean Med Sci. 1994; 9:458–465. PMID: 7786441.

12. Lee SN, Nam EM, Cha JH, et al. Adult T-cell leukemia/lymphoma with features of CD30-positive anaplastic large cell lymphoma: a case report. J Korean Med Sci. 1997; 12:364–368. PMID: 9288638.
13. Jeon HJ, Lee MJ, Jeong YK, Lee DM, Oh YK, Kim CW. Adult T-cell leukemia/lymphoma with lymphopenia in a Korean. J Korean Med Sci. 2000; 15:233–239. PMID: 10803704.
14. Ryu JH, Park JS, Hong SI, Son SJ, Suh CI. A case adult T-cell leukemia/ lymphoma. Korean J Dermatol. 2002; 40:295–299.
15. Jung JY, Lee JH, Lee KH. A case of adult T-cell leukemia/ lymphoma. Korean J Dermatol. 2007; 45:58–62.
Fig. 1
Dermatic presentation and radiologic evaluations. The patient showed multiple erythematous patches on his back (A). CT scan revealed multiple enlarged cervical lymph nodes (B) with remarkable hepatosplenomegaly (C).
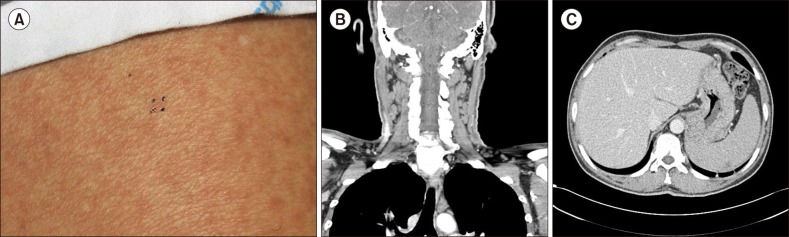
Fig. 2
Microscopic and immunohistochemical staining features. Medium-sized atypical lymphoid cells totally replaced a lymph node (A) (Haematoxylin-eosin stain, ×400) with strong positivity of CD3, CD4, and Ki-67 labeling index (B–D). On the skin biopsy, atypical lymphoid cells showed typical epidermotropism (E) (Haematoxylin-eosin stain, ×200), and massive perivascular infiltration (F) (Haematoxylin-eosin stain, ×200) resembling Mycosis fungoides. A strong positivity of CD4 and Ki-67 labeling index were shown (G, H). Atypical lymphoid cell infiltration was evident in his liver biopsy (I) (Haematoxylin-eosin stain, ×100) with strong CD4 reactivity (J). The same findings were noted in his bone marrow biopsy (K) (Haematoxylin-eosin stain, ×200) with CD4 immunohistochemical study (L). The HTLV-1 infected ‘flower cells’ were apparent in his peripheral blood smear (Wright-Giemsa ×1,000) (M–P).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download