Abstract
Background
Continuous infusion of factor VIII (FVIII) is a more cost-effective method for treating hemophilia A than intermittent bolus injection. However, there is currently no specific data in Korea about the progress of in vitro FVIII coagulant activity (FVIII:C) after reconstitution from its lyophilized form.
Methods
Three commercial FVIII concentrate products (two recombinant FVIII and one plasma-derived) were used. In vitro FVIII:C was measured at 0, 2, 4, 6, and 8 hours following reconstitution in both the indoor light-exposed and light-shielded groups.
Results
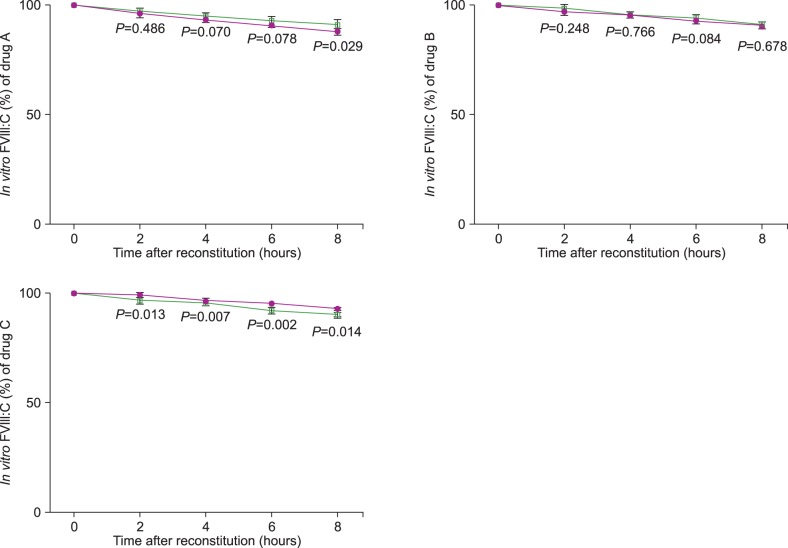
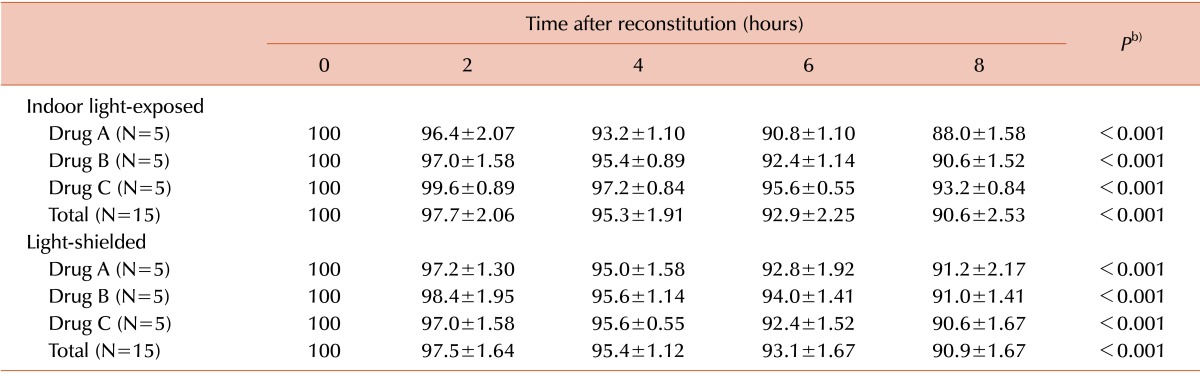
For the three drugs, in vitro FVIII:C decreased over the 8 hours following reconstitution (P<0.001). The decline of FVIII:C was linear (P<0.001). In vitro FVIII:C for the indoor light-exposed groups was 95.3±1.9% and 90.6±2.5% after 4 and 8 hours following reconstitution, respectively, compared to baseline activity. In the light-shielded group, FVIII:C was 95.4±1.1% and 90.9±1.7% of the baseline activity after 4 and 8 hours, respectively. There was no statistical difference between FVIII:C in the indoor light-exposed and light-shielded groups (P=0.849).
Conclusion
In vitro FVIII:C decreased after reconstitution, but activity was maintained at over 90% of the baseline value during 8 hours. Exposure to indoor light did not accelerate the loss of FVIII:C over the experimental time. This result indicates that CI with FVIII is available in 8-hour intervals, with no indoor light-exposure precautions needed.
In the management of patients with hemophilia A, continuous infusion (CI) of factor VIII (FVIII) is a more cost-effective method than bolus injection (BI), because it prevents unnecessary high peak of FVIII coagulant activity (FVIII:C), to maintain the desired level of FVIII from a pharmacological perspective [1, 2]. In practice, the benefits of using CI of FVIII was confirmed compared to BI, especially in cases of major surgery in patients with hemophilia A.
The nadir level of FVIII in the CI group was higher than that in the BI group [3, 4], and dangerous drops of FVIII levels below 0.3% were more frequent in patients receiving BI than those receiving CI [3]. Hemoglobin levels were also higher with CI than with BI, and the BI group required more blood transfusions than the CI group [3]. Major bleeding complications developed only in the BI group [3]. The CI group required 30-36% lower doses of FVIII than did the patients receiving BI [3, 5]. Although some reports have raised concerns about the possibility of inhibitor development, recent large-scale studies have shown no increased rate of inhibitor development in CI [6, 7]. Despite the aforementioned advantages of CI, there is currently no specific guideline for how frequently a FVIII product should be changed over the course of one day, or of the indoor light-shielding requirements for FVIII-containing vessels or syringes in Korea.
Acquiring absolute hemostasis in patients with hemophilia A depends on maintaining the desired FVIII:C level. There are two crucial factors for determining FVIII:C - individual in vivo recovery of FVIII:C, and in vitro FVIII:C in the syringe prior to injection. In vitro FVIII:C is particularly important in CI, since FVIII concentrate is kept in the syringe for several hours prior to entering the patient's body. Thus in this study, in vitro FVIII:C after reconstitution was estimated by using three commercial FVIII products in Korea; we also discuss the results and how they compare to previous data.
Three commercial FVIII products were used in this study for in vitro FVIII:C estimation: two recombinant FVIII concentrates, GreenGene F and Advate, and one plasma-derived product, GreenMono. The two recombinant FVIII concentrates are hereby described as drug A and B, respectively, while the plasma-derived product is designated as drug C.
Drug A (500 IU per vial) was reconstituted in 4 mL of sterile distilled water, according to manufacturer's instructions. After 30 minutes of stabilizing, 4 mL of the drug A diluent was further diluted 100-fold in sterile distilled water. Drug B (250 IU per vial) was diluted in 5 mL of sterile distilled water, according to the manufacturer's guidelines, allowing 30 minutes for reconstitution. Drug B was then further diluted 80-fold in sterile distilled water. Drug C (250 IU per vial) was diluted in 10 mL of sterile distilled water, based on the manufacturer's instructions. After 30 minutes, drug C was further diluted 20-fold in sterile distilled water. Each solution of drug A, B, and C was divided into 50 polyethylene tubes, each containing 1 mL of fluid. These were then divided into two groups: indoor light-exposed and light-shielded. The general outline for preparing the experiment is shown in Fig. 1.
In vitro FVIII:C was measured at five time points (0, 2, 4, 6, and 8 hours after reconstitution) for both the indoor light-exposed and light-shielded treatments of each of the three commercial drugs. The estimation of in vitro FVIII:C was performed with a one-stage clotting assay using a STA-R evolution automatic coagulation analyzer (Diagnostica Stago). The mean value of FVIII:C estimated at 0 hours was defined as baseline (100%) activity.
To analyze the changes of in vitro FVIII:C over time (0, 2, 4, 6, and 8 hours after reconstitution), repeated measures of ANOVA analysis were used. To compare the mean values of FVIII:C according to the time after reconstitution between the indoor light-exposed and light-shielded groups, independent t-tests with Bonferroni corrections were used. To demonstrate statistical significance of decreases in FVIII:C, an ANOVA test with the Bonferroni method was performed. Data are described as means±SDs. SPSS version 21.0 software (Knowledge Dynamics, Canyon Lake, TX) was used for statistical analysis. P values of <0.05 were considered statistically significant. In the case of Bonferroni corrections, the P values of <0.05/N were considered statistically significant.
All the in vitro FVIII:C values at 0, 2, 4, 6, and 8 hours were converted into percentages of activity, based on the baseline activity measurement at 0 hours. For each of the three drugs, in vitro FVIII:C was decreased as time increased (from 0 to 8 hours after reconstitution) in both the indoor light-exposed and light-shielded groups (P<0.001). The decline of FVIII:C is linear (P<0.001). In vitro FVIII:C were 95.3±1.9% and 90.6±2.5% at 4 and 8 hours after reconstitution, respectively, in the indoor light-exposed group. In light-shielded group, FVIII:C at 4 and 8 hours after reconstitution were 95.4±1.1% and 90.9±1.7%, respectively. The respective FVIII:C values and the P values of the ANOVA tests between the time points are described in Table 1. For each of the three drugs in the indoor light-exposed group, the in vitro FVIII:C decreased after 2 hours (P=0.018). In the light-shielded group, in vitro FVIII:C also decreased after 2 hours for each drug (P<0.001).
There was no statistical difference between FVIII:C in the indoor light-exposed and light-shielded groups for all three drugs (P=0.849). There was also no difference between the degree of loss in FVIII:C over time for all three drugs in both light treatments (P=0.957). There was no statistical difference in FVIII:C between the indoor light-exposed and light-shielded groups for each of the three drugs as well. The progress of FVIII:C and the P values are shown in Fig. 2 (by Bonferroni method, P<0.0125).
Generally, infusion of FVIII concentrate according to the body weight (IU/kg) is known to raise the patient's FVIII:C by approximately 2 IU/dL. However, the drug should be supplemented according to the patient's response, due to considerable inter- and intra-patient variability in pharmacokinetics (PK) [8, 9]. There has been an emphasis on individual dosing of clotting factors using PK, to avoid the inadequate management and under-treatment of patients who require additional dosage, and to prevent potential overtreatment of the patients who require lower doses, which results in wasting of an expensive product [8, 9]. It is possible to calculate the velocity of CI if we obtain the level of FVIII from patients with hemophilia A during treatment [8, 9].
Several factors influence individual PK in the use of FVIII for treating patients with hemophilia A without inhibitor formation - product type, the patient's age, weight, body surface area, plasma volume, endogenous von Willebrand factor level, blood type, and hematocrit [10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20]. Thus, the indicators of PK change with advanced age, and may vary depending on the circumstances of each hemophilia patient. Besides the aforementioned factors, the in vitro FVIII:C that has been stored in a syringe prior to entering the patient's body must be considered for CI. It is a crucial factor because FVIII concentrate is reconstituted and stored in the syringe pump for several hours before infusion.
Several studies have reported the in vitro FVIII:C of various products. In the case of Bioclate, in vitro FVIII:C after reconstitution was maintained at over 75% of baseline activity for a period of 3-7 days [21]. In the case of Recombinate, in vitro FVIII:C remained stable during simulated CI for 96 hours [22]. For ADVATE rAHF-PFM, in vitro FVIII:C retained an average of 92% of its baseline activity over 24 hours after reconstitution [23] and maintained 83% or more of its activity at 24 or 48 hours in simulated CI [24].
In this study, the decline of in vitro FVIII:C was linear from 0-8 hours after reconstitution. In other words, in vitro FVIII:C loss begins immediately after reconstitution and consistently drops during the course of CI. However, the degree of in vitro FVIII:C loss does not seem to be clinically severe in 8 hours, because average FVIII:C was sustained at over 95% and 90% at 4 and 8 hours after reconstitution, respectively. This result suggests that CI with a FVIII concentrate is possible in 8-hour intervals in practice. Additionally, medical personnel should note that FVIII:C can decrease to under 90% of the baseline value after more than 8 hours, particularly because lower levels of FVIII in treatment cannot establish absolute hemostasis in patients with hemophilia A.
In comparison to the results of previous reports [21, 22, 23, 24, 25], the level of in vitro FVIII:C seems to be slightly lower in this study. We suggest that the experimental methods could have influenced the results. 1) FVIII was diluted after reconstitution and subsequently kept for several hours for the experiment, while in previous studies, undiluted reconstituted FVIII was kept for several hours and diluted just prior to experimentation [25]. 2) Estimation of FVIII:C required dilution of FVIII with sterile water in this study, while past reports have used normal saline/plasma of patients with severe hemophilia A [25], or did not dilute the reconstituted FVIII [22, 23, 24]. 3) We also considered whether the efficacy would be affected by the year the product was released or by its expiration date. Further study is needed to determine whether these factors are important.
In a previous report, the activity of reconstituted FVIII was diminished to approximately 30% of its baseline activity after 10 hours of daylight exposure, while shielding in foil wrap prevented the harmful effects of daylight [22]. However, persistent exposure to natural daylight is very rare in a practical clinical environment, especially for patients with hemophilia A who are under CI of FVIII, which is why indoor light was used in this study. There was no difference between FVIII:C in the indoor light-exposed or light-shielded groups during the experimental time frame of 8 hours. This result shows that CI using FVIII concentrate over a period of 8 hours does not require additional protection from indoor light in hospital rooms.
In conclusion, in vitro FVIII:C after reconstitution consistently decreases in the syringe prior to injection into the patient's body during CI, a fact that should be noted by the medical personnel. In vitro FVIII:C decreased after reconstitution, but the activity remained at over 90% of baseline activity, which is not clinically severe over an 8-hour time frame. Further, exposure to indoor light did not accelerate the loss of FVIII:C during 8 hours. These results indicate that FVIII CI is an available treatment option in 8-hour intervals in practice and indoor light-shielding precautions are not needed to safeguard FVIII:C.
References
1. Batorova A, Martinowitz U. Continuous infusion of coagulation factors: current opinion. Curr Opin Hematol. 2006; 13:308–315. PMID: 16888434.

3. Batorova A, Martinowitz U. Intermittent injections vs. continuous infusion of factor VIII in haemophilia patients undergoing major surgery. Br J Haematol. 2000; 110:715–720. PMID: 10997985.

4. Hathaway WE, Christian MJ, Clarke SL, Hasiba U. Comparison of continuous and intermittent Factor VIII concentrate therapy in hemophilia A. Am J Hematol. 1984; 17:85–88. PMID: 6430068.

5. Bidlingmaier C, Deml MM, Kurnik K. Continuous infusion of factor concentrates in children with haemophilia A in comparison with bolus injections. Haemophilia. 2006; 12:212–217. PMID: 16643203.

6. Batorova A, Holme P, Gringeri A, et al. Continuous infusion in haemophilia: current practice in Europe. Haemophilia. 2012; 18:753–759. PMID: 22530687.

7. Auerswald G, Bade A, Haubold K, Overberg D, Masurat S, Moorthi C. No inhibitor development after continuous infusion of factor concentrates in subjects with bleeding disorders undergoing surgery: a prospective study. Haemophilia. 2013; 19:438–444. PMID: 23279056.

8. Berntorp E, Bjorkman S. The pharmacokinetics of clotting factor therapy. Haemophilia. 2003; 9:353–359. PMID: 12828668.

9. Shapiro AD, Korth-Bradley J, Poon MC. Use of pharmacokinetics in the coagulation factor treatment of patients with haemophilia. Haemophilia. 2005; 11:571–582. PMID: 16236106.

10. Aronstam A, McLellan DS, Wassef M, Mbatha PS. Effect of height and weight on the in vivo recovery of transfused factor VIII C. J Clin Pathol. 1982; 35:289–291. PMID: 6802879.

11. Fukui H, Yoshioka A, Shima M, et al. Clinical evaluation of recombinant human factor VIII (BAY w 6240) in the treatment of hemophilia A. Int J Hematol. 1991; 54:419–427. PMID: 1756252.
12. Morfini M, Longo G, Messori A, Lee M, White G, Mannucci P. The Recombinate Study Group. Pharmacokinetic properties of recombinant factor VIII compared with a monoclonally purified concentrate (Hemofil M). Thromb Haemost. 1992; 68:433–435. PMID: 1448776.

13. Fijnvandraat K, Peters M, ten Cate JW. Inter-individual variation in half-life of infused recombinant factor VIII is related to pre-infusion von Willebrand factor antigen levels. Br J Haematol. 1995; 91:474–476. PMID: 8547097.

14. Fijnvandraat K, Berntorp E, ten Cate JW, et al. Recombinant, B-domain deleted factor VIII (r-VIII SQ): pharmacokinetics and initial safety aspects in hemophilia A patients. Thromb Haemost. 1997; 77:298–302. PMID: 9157585.

15. White GC 2nd, Courter S, Bray GL, Lee M, Gomperts ED. The Recombinate Previously Treated Patient Study Group. A multicenter study of recombinant factor VIII (Recombinate) in previously treated patients with hemophilia A. Thromb Haemost. 1997; 77:660–667. PMID: 9134639.
16. Vlot AJ, Mauser-Bunschoten EP, Zarkova AG, et al. The half-life of infused factor VIII is shorter in hemophiliac patients with blood group O than in those with blood group A. Thromb Haemost. 2000; 83:65–69. PMID: 10669157.
17. Mondorf W, Klinge J, Luban NL, et al. Low factor VIII recovery in haemophilia A patients without inhibitor titre is not due to the presence of anti-factor VIII antibodies undetectable by the Bethesda assay. Haemophilia. 2001; 7:13–19. PMID: 11136375.

18. Yoshioka A, Shima M, Fukutake K, Takamatsu J, Shirahata A. Safety and efficacy of a new recombinant FVIII formulated with sucrose (rFVIII-FS) in patients with haemophilia A: a long-term, multicentre clinical study in Japan. Haemophilia. 2001; 7:242–249. PMID: 11380627.

19. Windyga J, Rusen L, Gruppo R, et al. BDDrFVIII (Moroctocog alfa [AF-CC]) for surgical haemostasis in patients with haemophilia A: results of a pivotal study. Haemophilia. 2010; 16:731–739. PMID: 20412322.

20. Takedani H. Continuous infusion during total joint arthroplasty in Japanese haemophilia A patients: comparison study among two recombinants and one plasma-derived factor VIII. Haemophilia. 2010; 16:740–746. PMID: 20398072.

21. Belgaumi AF, Patrick CC, Deitcher SR. Stability and sterility of a recombinant factor VIII concentrate prepared for continuous infusion administration. Am J Hematol. 1999; 62:13–18. PMID: 10467271.

22. Parti R, Ardosa J, Yang L, Mankarious S. In vitro stability of recombinant human factor VIII (Recombinate). Haemophilia. 2000; 6:513–522. PMID: 11012695.

23. Parti R, Schoppmann A, Lee H, Yang L. Stability of lyophilized and reconstituted plasma/albumin-free recombinant human factor VIII (ADVATE rAHF-PFM). Haemophilia. 2005; 11:492–496. PMID: 16128893.

24. Fernandez M, Yu T, Bjornson E, Luu H, Spotts G. Stability of ADVATE, Antihemophilic Factor (Recombinant) Plasma/Albumin-Free Method, during simulated continuous infusion. Blood Coagul Fibrinolysis. 2006; 17:165–171. PMID: 16575253.

25. Schulman S, Gitel S, Martinowitz U. Stability of factor VIII concentrates after reconstitution. Am J Hematol. 1994; 45:217–223. PMID: 8296792.

Fig. 1
The general outline of the experimental procedure is shown. Estimation of in vitro FVIII:Ca) was performed using three commercial products, divided into indoor light-exposed and light-shielded experimental groups. a)Factor VIII coagulant activity.
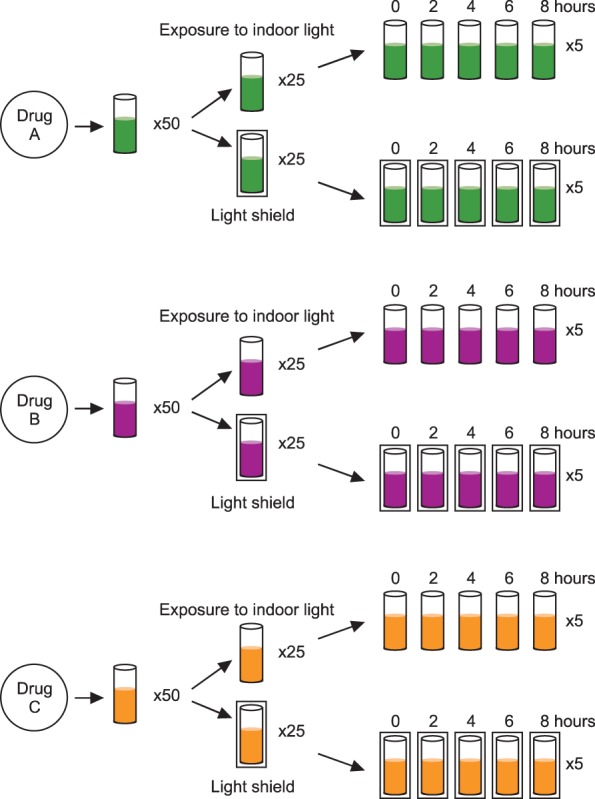
Fig. 2
Comparison of in vitro FVIII:Ca) for each drug between the indoor light-exposed and light-shielded groups, during a period of 8 hours. The filled circles represent the FVIII:C in indoor light-exposed groups, while the empty rectangles represent the FVIII:C in light-shielded group. The values of P<0.0125 were considered as statistically significant on using the Bonferroni method. a)Factor VIII coagulant activity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download