Abstract
Indeterminate dendritic cell tumor (IDCT) is a proliferation of CD1a+, S100+ and langerin- histiocytes with a generally benign course. Here, we describe a case of a 90-year-old male who developed skin lesions on his scalp mimicking angiosarcoma and lymphadenopathy. He died six months after the onset of skin lesions despite of months' radiotherapy. Pathological examination ruled out scalp angiosarcoma and showed a high Ki-67 index. The appearance of skin lesions and lymphadenopathy led to challenges in diagnosis and the development of a treatment plan.
Indeterminate dendritic cell tumor (IDCT) is a rare neoplasm sharing some common features with Langerhans cell histiocytosis with respect to morphology and immunophenotype, but lacking Birbeck granules characteristic of Langerhans cells1. IDCT can occur as multiple solid red, yellow, or reddish-brown papulonodules, or, less commonly, as a solitary lesion. The clinical course of IDCT is usually benign, IDCT-related mortality was hardly seen in literature and there is no established treatment protocol to follow1. Here, we report a rare case of IDCT which resembled angiosarcoma of the scalp with an aggressive clinical course.
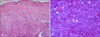
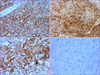
A 90-year-old male with a three month history of asymptomatic skin lesions on his scalp attended our dermatology department. Skin examination showed multiple violaceous confluent papules, nodules and plaques with a central haemorrhagic crust on his scalp (Fig. 1). Moreover, an enlarged preauricle lymph node (1.5×1.5 cm) was noted. The patient was otherwise well. The results of routine laboratory studies were negative or within normal limits. The pathological evaluation revealed hyperkeratosis of the epidermis, with multiple tumor nodules with central necrosis infiltrated in the dermis. The medium-sized tumor cells had abundant eosinophilic cytoplasm. Reniform nuclei and nuclear grooves were occasionally observed. Multinucleate giant cells and eosinophils were absent (Fig. 2). Immunohistochemical analyses showed that the tumor cells were positive for CD1a, S100, CD31, partially positive for CD68 (PGM1), and negative for Langerin, CD34, FXIIIa, Fli-1, HMB45, A103, EMA and PCK (Fig. 3). The Ki-67 proliferation index was 35%. A diagnosis of IDCT was made.
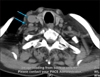
We thought this patient's lymphadenopathy were no malignant at first. Because IDCT never shows a metastatic feature. So, local electron beam therapy (20 Gy/10 fractions) was initiated to his scalp lesions, and nearly 80% of lesions regressed. Two months later, palpable non-tender nodes developed in the patient's right neck and multiple painless, firm, subcutaneous nodules with poor mobility were found on his scalp, face and neck. Computed tomography scan showed multiple nodules in his bilateral parotid glands region, right carotid sheath region and mediastinum, and enlarged lymph nodes were absent in his abdomen (Fig. 4). A subsequent intensity modulated radiation therapy was delivered to his neck region and mediastinum with 40 Gy/10 fractions separately. The patient's condition continued to deteriorate and he died due to respiratory and circulatory failure three months later.
The pathogenesis of IDCT remains unclear. It was not until the publication of the 2008 World Health Organization classification of tumors of hematopoietic and lymphoid tissues that this entity was categorized as “intermediate dendritic cell tumor”. The terms IDCT, intermediate dendritic cell tumor, indeterminate cell tumor, and indeterminate cell histiocytosis all refer to the same entity in the literature. IDCT skin lesions generally occur on the face, trunk and extremities, and usually present as red to brown papulonodules, solitary lesions have also been observed in a limited number of cases, and visceral involvement is rare1. Histologically, IDCT is characterized by a dermal infiltrate composed of cells with abundant eosinophilic cytoplasm and oval-to-indented nuclei that resemble Langerhans cells. Immunohistochemical staining makes the distinction from other types of histiocytosis possible. The tumor cells in IDCT are consistently S100 and CD1a positive, but lack langerin expression which is used as surrogate marker for the presence of Birbeck granules. The formation of Birbeck granules is dependent on the invaginations of endocytic structures mediated by langerin/CD207, a type II transmembrane protein2. A lack of langerin expression is remarkably matched with the absence of Birbeck granules in clinical cases1.
The origin of indeterminate cells is debated. It has been proposed that they are precursor cells of mononuclear phagocytic and dendritic cells based on their phenotypic characteristics from both lineages. Brown et al.3 observed ETV3-NCOA2 clonal translocations in three IDCT cases, which were absent in the control groups of other types of histiocytosis, a finding that may suggest a particular pathogenesis of IDCT.
There is no age or sex predilection in IDCT1. The patient in this case is the oldest described in the literature to the best of our knowledge.The location of his skin lesions on the scalp differed from other cases of IDCT, and the lesions were highly reminiscent of scalp angiosarcoma. However, the histological changes did not indicate angiosarcoma, which is characterized by pleomorphic and hyperchromatic endothelial cells and the formation of ill-defined vascular spaces. The distant metastases and subsequent fatal prognosis in this case suggests that IDCT can be aggressive in rare cases. Deng et al.4 reported an IDCT patient with skin lesions located on the scalp. This patient had a favorable prognosis; although, in this case the lesions had the appearance of melanoma. Vener et al.5 reported a case of IDCT where the patient developed acute myeloblastic leukemia (AML) after six years of intermittent chemotherapy. However, it is difficult to establish whether the development of AML was associated with IDCT due to the application of chemotherapies and the relatively large time span between the onset of these two diseases.
The Ki-67 index of 35%, which was higher than in previously reported cases67, might hint at the aggressive clinical course in this case. Rezk et al.1 reported an IDCT patient with B-cell gene rearrangement who experienced an aggressive clinical course. Although the patient had a history of follicular lymphoma, there was no evidence of the coexistence of these two diseases. Unfortunately, the Ki-67 index was not described. Our case suggests that IDCT could follow a highly malignant course. Advanced age, distant metastases and a high Ki-67 index are potential risk factors.
CD31, also known as platelet endothelial cell adhesion molecule 1, is a transmembrane glycoprotein expressed by endothelial cells and a variety of hematopoietic cells. It is a sensitive and specific marker for vascular differentiation and is consistently positive in angiosarcoma. In some cases, positive CD31 staining has led to misdiagnosis when it was not considered that a variety of hematopoietic cells are CD31 positive, including macrophages and histiocytes8. Sporadic cases of xanthogranuloma, reticulohistiocytoma, Langerhans cell histiocytosis and xanthoma have been found to be CD31 positive9, which makes histiocytosis a consideration in differential diagnosis of vascular neoplasms.
There is no treatment protocol for IDCT to follow due to the rarity of this disease. Successful treatment of multiple IDCT lesions with electron beam10, ultraviolet B11, thalidomide12, and methotrexate13 has been reported. Surgical excision is more appropriate for solitary lesions4. The most impressive feature of the case reported here was the remarkable progress of the illness, which suggests that some cases of IDCT require aggressive treatment for the benefit of the patient.
Figures and Tables
Fig. 1
Multiple violaceous confluent papules, nodules and plaques with a central hemorrhagic crust mimicking angiosarcoma.
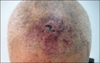
Fig. 2
(A) Hyperkeratosis of the epidermis and tumor nodules in dermis (H&E, ×100). (B) Medium-sized tumor cells with abundant eosinophilic cytoplasm. Reniform nuclei and delicate nuclear grooves are indicated by white arrows (H&E, ×400).
References
1. Rezk SA, Spagnolo DV, Brynes RK, Weiss LM. Indeterminate cell tumor: a rare dendritic neoplasm. Am J Surg Pathol. 2008; 32:1868–1876.
2. Valladeau J, Dezutter-Dambuyant C, Saeland S. Langerin/CD207 sheds light on formation of birbeck granules and their possible function in Langerhans cells. Immunol Res. 2003; 28:93–107.

3. Brown RA, Kwong BY, McCalmont TH, Ragsdale B, Ma L, Cheung C, et al. ETV3-NCOA2 in indeterminate cell histiocytosis: clonal translocation supports sui generis. Blood. 2015; 126:2344–2345.

4. Deng A, Lee W, Pfau R, Harrington A, DiGiovani J, Prickett KA, et al. Primary cutaneous Langerhans cell sarcoma without Birbeck granules: indeterminate cell sarcoma? J Cutan Pathol. 2008; 35:849–854.

5. Vener C, Soligo D, Berti E, Gianelli U, Servida F, Ceretti E, et al. Indeterminate cell histiocytosis in association with later occurrence of acute myeloblastic leukaemia. Br J Dermatol. 2007; 156:1357–1361.

6. Mo X, Guo W, Ye H. Primary indeterminate dendritic cell tumor of skin correlated to mosquito bite. Medicine (Baltimore). 2015; 94:e1443.

7. Chen M, Agrawal R, Nasseri-Nik N, Sloman A, Weiss LM. Indeterminate cell tumor of the spleen. Hum Pathol. 2012; 43:307–311.

8. McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001; 25:1167–1173.
9. Vanchinathan V, Mirzamani N, Kantipudi R, Schwartz EJ, Sundram UN. The vascular marker CD31 also highlights histiocytes and histiocyte-like cells within cutaneous tumors. Am J Clin Pathol. 2015; 143:177–185.

10. Malhomme de la Roche H, Lai-Cheong JE, Calonje E, Davies M, Morris S, Whittaker SJ. Indeterminate cell histiocytosis responding to total skin electron beam therapy. Br J Dermatol. 2008; 158:838–840.

11. Ishibashi M, Ouchi T, Tanikawa A, Ishiko A. Indeterminate cell histiocytosis successfully treated with ultraviolet B phototherapy. Clin Exp Dermatol. 2008; 33:301–304.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download