Abstract
Background
Objective
Methods
Results
Figures and Tables
Fig. 2
Disease severity classified by psoriasis area and severity index (PASI), body surface area (BSA), and dermatology life quality index (DLQI). Overall mean±standard deviation (SD) are presented below the title. Each n (%) of the classified group is presented.
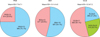
Table 1
Demographics

Values are presented as total number only, number (%), or mean±standard deviation. Total number does not always represent 100% of the study population because of missing values. Percentages of subgroup (by gender) were based on the total number of subjects in each PASI group per subgroup. PASI: psoriasis area and severity index, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure. *p-value<0.05.
Table 2
Types of psoriasis

| Classification | Overall (n=1,260) |
|---|---|
| Plaque | 1,081 (85.8) |
| Guttate | 106 (8.4) |
| Pustular | 66 (5.2) |
| Erythrodermic | 44 (3.5) |
| Annular | 24 (1.9) |
| Inverse | 4 (0.3) |
Table 3
Location of involved lesions
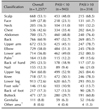
Table 4
Past and current therapies
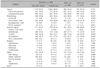
Table 5
Adjusted effect of characteristics on PASI ≥10 based on multiple logistic regression model (n=1,086)

One hundred seventy-four patients with missing data were excluded from the logistic regression analysis. PASI: psoriasis area and severity index, Ref.: reference for odds ratio, OR: odds ratio, CI: confidence interval, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure. *p-value<0.05.
Table 6
Comparing the degree of satisfaction with treatment

Notes
CONFLICTS OF INTEREST Dr. Hae Jun Song has participated as an advisory board member and has conducted clinical trials for Janssen and has received speaking fees or research support from Janssen. Dr. Chul Jong Park, Dr. Tae Yoon Kim, Dr. Yong Beom Choe, Dr. Seok-Jong Lee, Dr. Nack In Kim, Dr. Jae We Cho, Dr. Jie Hyun Jeon, Dr. Min Soo Jang have no conflict of interest to declare. Dr. Jai Il Youn has conducted clinical trial for Pfizer, Janssen, Lilly, Leo. Dr. Myung Hwa Kim, Dr. Joonsoo Park, Dr. Ki Ho Kim, Dr. Byung Soo Kim have no conflict of interest to declare. Dr. Sang Woong Youn has served as an advisor, received speaker honoraria, and participated in clinical trials for Janssen. Dr. Joo-Heung Lee has no conflict of interest to declare. Dr. Min-Geol Lee has conducted clinical trials for Pfizer, Novartis, Eli Lilly, and Janssen and has received speaking fees from Janssen. Dr. Sung Ku Ahn, Dr. Young Ho Won, Dr. Seok Kweon Yun, Dr. Bong Seok Shin have no conflict of interest to declare. Dr. Seong Jun Seo has conducted clinical trials for Amore, Regeneron, Novartis, Roche. Dr. Ji Yeoun Lee, Dr. Kwang Joong Kim have no conflict of interest to declare. Dr. Young Suck Ro has conducted post-marketing surveillance for Janssen. Mr. Dae Young Yu and Mr. Youngdoe Kim are employee of Janssen Korea. Dr. Jee-Ho Choi has conducted clinical trials for Janssen.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download