Abstract
Bowenoid papulosis (BP) is a disease that mainly occurs in sexually active young adults. The patients present with multiple pigmented papular lesions with a verrucous surface on the anogenital area. Although extragenital BP is usually associated with concomitant genital involvement, a few cases of isolated extragenital BP have been reported. However, to our knowledge, BP of the nipple has not yet been reported in the medical literature. We report the case of a 32-year-old woman who presented with isolated extragenital BP of the nipple caused by human papillomavirus-16.
Bowenoid papulosis (BP) is an infectious disease caused by human papillomavirus (HPV). Although various HPV types are related to BP, HPV-16 is the most common causative agent. Histopathologically, BP resembles Bowen disease. Most previously reported lesions were observed on the genital area; however, they may also occur elsewhere. Although extragenital BP is usually accompanied by concomitant anogenital lesions, 11 cases of isolated extragenital BP have been reported. To our knowledge, isolated extragenital BP is a very rare disease, especially, BP of the nipple, as in our patient, has not been reported in the medical literature. Therefore, we report an interesting case of BP along with a review of the literature.
A 32-year-old Korean woman visited the dermatologic department of our hospital for the evaluation of a verrucous lesion on her right nipple. The lesion occurred 1 year ago and had been progressively increasing in size. She had no subjective symptoms including pain, tenderness, and itchiness. She had no family or personal history of skin cancer or other medical diseases such as diabetes mellitus, cardiovascular diseases, and liver diseases. She was not married.
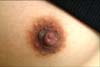
Physical examination revealed a 0.7×0.8 cm dark brownish verrucous papule on the right nipple (Fig. 1). However, the lesion was not associated with concomitant genital, oral, and periungual involvement.
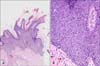
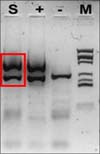
Histopathological examination revealed parakeratosis, acanthosis, and papillomatosis of the epidermis (Fig. 2). High-power (×200) magnification revealed atypical and dyskeratotic keratinocytes with mitotic figures and loss of orderly maturation throughout the thickened epidermis. Polymerase chain reaction revealed the presence of HPV-16 DNA sequences (MyHPV chip; Biomedlab, Seoul, Korea) (Fig. 3). The histologic features were most consistent with BP caused by HPV-16.
After the lesion was diagnosed as a case of BP, it was successfully treated with electrodesiccation. No recurrence of the lesion has been observed for 5 years.
BP is a rare, sexually transmitted disorder caused by HPV; it is characterized by the occurrence of lesions on the genitals of men and women. The lesions are reddish brown or violet, small, solid, raised, and sometimes velvety. HPV has been defined as a causative agent of BP. Various types of HPV have been detected in causing BP, including HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-42 to HPV-45, HPV-51, HPV-52, and HPV-56. HPV-16 is the most common; HPV-16, HPV-18, and HPV-33 are considered the most oncogenic types. However, HPV-16 is usually detected in several diseases, including anogenital warts, bowenoid papulosis, Bowen disease, and invasive cancer.
BP was first described by Lloyd1 in 1970, in a patient with multicentric, pigmented Bowen disease of the groin. The term BP was initially used by Wade et al.2 in 1978 to describe multiple genital verrucous papules that histologically resembled squamous cell carcinoma in situ. Extragenital BP associated with concurrent anogenital involvement was first described by Rüdlinger et al.3 in 1989. They reported a case of HPV-35-positive BP on the right ring finger, with concomitant anogenital involvement. Although there had been many cases of extragenital BP, these cases were always accompanied with concomitant anogenital BP.
Isolated extragenital BP was first described by Bart4 in 1984. He reported on a 35-year-old woman who presented with BP of the chin without involvement at any other site. Thereafter, several cases have been reported in the medical literature (Table 1)4,5,6,7,8,9,10,11,12,13. Of all these cases, 4 were BP of the neck; 3 were of the chin; and the others were of the abdomen, elbow, finger, and toe web.
As extragenital BP is usually associated with concomitant anogenital BP, it may be caused by autoinoculation from the preceding genital BP13. However, as isolated extragenital BP has no concomitant lesions, the possibility of inoculation of extragenital sites through contaminated implements or subclinical genital infections has been suggested8. Although no genital lesions were found in our patient, this does not rule out a previous or current subclinical infection being a possible source.
The patient described by Fader et al.7 was 48-year-old homosexual man with acquired immune deficiency syndrome. Their case may suggest that immunosuppression may affect the development of extragenital BP. In addition, Papadopoulos et al.11 reported a patient with human immunodeficiency virus infection and Purnell et al.12 reported on a patient with CD4+ lymphocytopenia with BP, which seem to indicate a relation between BP and an immunocompromised status. However, our patient did not have medical diseases or immune abnormalities, and the other previously reported patients had no combined diseases. Therefore, the relation of BP with immunosuppression is unclear.
The course of BP is variable, ranging from progression to cancer and chronic diseases to spontaneous regression. BP may be considered a form of low-grade squamous cell carcinoma in situ. Progression to Bowen disease and invasive squamous cell carcinoma has rarely been reported14,15,16,17. Therefore, patients with BP should be monitored carefully because of the risk of squamous cell carcinoma development.
Figures and Tables
Fig. 2
Histopathological findings. (A) Parakeratosis, acanthosis, and papillomatosis of the epidermis (H&E, ×200). (B) Atypical and dyskeratotic keratinocytes with mitotic figures and loss of orderly maturation scattered throughout the epidermis (H&E, ×40).
References
3. Rüdlinger R, Grob R, Yu YX, Schnyder UW. Human papillomavirus-35-positive bowenoid papulosis of the anogenital area and concurrent human papillomavirus-35-positive verruca with bowenoid dysplasia of the periungual area. Arch Dermatol. 1989; 125:655–659.

5. Grussendorf-Conen EI. HPV-16 induzierte pigmentierte bowenoide Papulose am Hals. Akt Dermatol. 1988; 14:317–319.
6. Grob JJ, Zarour H, Jacquemier J, Hassoun J, Bonerandi JJ. Extra-anogenital HPV16-related bowenoid papulosis. Genitourin Med. 1991; 67:18–20.

7. Fader DJ, Stoler MH, Anderson TF. Isolated extragenital HPV-thirties-group-positive bowenoid papulosis in an AIDS patient. Br J Dermatol. 1994; 131:577–580.

8. Baron JM, Rübben A, Grussendorf-Conen EI. HPV 18-induced pigmented bowenoid papulosis of the neck. J Am Acad Dermatol. 1999; 40:633–634.

9. Johnson TM, Saluja A, Fader D, Blum D, Cotton J, Wang TS, et al. Isolated extragenital bowenoid papulosis of the neck. J Am Acad Dermatol. 1999; 41:867–870.

10. Olhoffer IH, Davidson D, Longley J, Glusac EJ, Leffell D. Facial bowenoid papulosis secondary to human papillomavirus type 16. Br J Dermatol. 1999; 140:761–762.
11. Papadopoulos AJ, Schwartz RA, Lefkowitz A, Tinkle LL, Jänniger CK, Lambert WC. Extragenital bowenoid papulosis associated with atypical human papillomavirus genotypes. J Cutan Med Surg. 2002; 6:117–121.

12. Purnell D, Ilchyshyn A, Jenkins D, Salim A, Seth R, Snead D. Isolated human papillomavirus 18-positive extragenital bowenoid papulosis and idiopathic CD4+ lymphocytopenia. Br J Dermatol. 2001; 144:619–621.

13. Oh SH, Lee JH, Lee KY, Lee KH. Isolated extragenital bowenoid papulosis of the toe-webs. Acta Derm Venereol. 2009; 89:212–213.
14. Obalek S, Jablonska S, Beaudenon S, Walczak L, Orth G. Bowenoid papulosis of the male and female genitalia: risk of cervical neoplasia. J Am Acad Dermatol. 1986; 14:433–444.

15. Bonnekoh B, Mahrle G, Steigleder GK. Transition to cutaneous squamous cell carcinoma in 2 patients with bowenoid papulomatosis. Z Hautkr. 1987; 62:773–776. 780–784.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download