Dear Editor:
Transcatheter arterial chemoembolization (TACE) is widely used as an approach for patients with hepatocellular carcinoma (HCC). Cutaneous complications related to TACE such as erythema, necrosis, and scarring may rarely occur1. This report describes the first known case of cutaneous metastasis of HCC following skin injury after TACE.
A 39-year old man had been diagnosed with HCC in January 2010. On the initial computed tomography (CT) image, a 7.8 cm sized tumor was noted. The patient was treated with TACE from February 2010 via the femoral artery. On a follow-up CT in May 2010, the tumor size decreased, however, daughter nodules were noted. The 7th TACE was performed via the internal mammary artery (IMA) due to the presence of collateral pathways. In November 2010, an 8th course of TACE of the IMA was performed. However, an erythematous to violaceous patch developed shortly after the infusion of chemotherapeutic agents and resulted in pigmentation and induration. In January 2011, the patient was referred to the dermatology clinic with two erythematous papules on the right chest that developed at the end of December 2010 (Fig. 1). The papules had developed within the pigmented induration, which was associated with skin injury after the TACE in November 2010.
Histopathologic examination of the erythematous papule showed asymmetrical, lobulated nests invading the dermis (Fig. 2A). The large cells had a polyhedral shape, eosinophilic cytoplasm, large central nuclei and prominent nucleoli (Fig. 2B). The specimen was negative for AFP (Fig. 2C). CD31 stain did not show evidence of hematogenous spread of tumor nests (Fig. 2D).
As the papules occurred on the scar and a series of examinations showed no other metastasis, the lesions were thought to be associated with the skin injury after TACE. The biopsy specimen was negative for AFP. However, positive staining for AFP has been reported to be present in 2 to 61% of HCC2. Expression of AFP can be weak, especially in small biopsies.
Tumor seeding of HCC after interventional procedures is a rare complication. Cutaneous tumor seeding of HCC along the tract of the needle used in the diagnosis has been reported3. The frequency of needle tract implantation after radiofrequency ablation ranges from 0 to 4.4%.3 Iatrogenic cutaneous tumor seeding by ultrasound-guided percutaneous ethanol injection has also been rarely reported4.
TACE via the IMA may be performed when collateral pathways are evident. Repetitive TACEs or large tumor sizes may be associated with the presence of collateral pathways. In our case, TACE-induced injury may have caused inflammatory changes or adhesion of the chest wall that helped the progression of the residual viable tumor along the liver surface to the skin. Adhesion between the HCC and other tissues including vessels such as the peritoneum and/or omentum after TACE has been shown in a laparoscopic study5.
We have presented a case of cutaneous metastasis from HCC following skin injury after TACE that was thought to be relevant to the cutaneous complication of the chemoembolization procedure. The potential cutaneous complications of TACE must be taken into consideration.
Figures and Tables
Fig. 1
Two, asymptomatic, erythematous papules and underlying pigmentation and wood plate-like induration on the right chest.
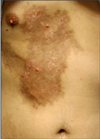
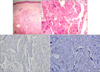
Fig. 2
(A) Asymmetrical, lobulated cords and nests invading the dermis (H&E, ×40). (B) The large epithelial cells of polyhedral shape showed granular, eosinophilic cytoplasm, large, vesicular, central nuclei and prominent nucleoli (H&E, ×400). (C) The specimen was negative for α-fetoprotein (AFP) (AFP, ×200). (D) CD31 stain did not show any evidence of hematogenous spread (CD31, ×200).
References
1. Arora R, Soulen MC, Haskal ZJ. Cutaneous complications of hepatic chemoembolization via extrahepatic collaterals. J Vasc Interv Radiol. 1999. 10:1351–1356.

2. Minervini MI, Demetris AJ, Lee RG, Carr BI, Madariaga J, Nalesnik MA. Utilization of hepatocyte-specific antibody in the immunocytochemical evaluation of liver tumors. Mod Pathol. 1997. 10:686–692.
3. Onodera H, Oikawa M, Abe M, Chida N, Kimura S, Satake K, et al. Cutaneous seeding of hepatocellular carcinoma after fine-needle aspiration biopsy. J Ultrasound Med. 1987. 6:273–275.

4. Chang S, Kim SH, Lim HK, Kim SH, Lee WJ, Choi D, et al. Needle tract implantation after percutaneous interventional procedures in hepatocellular carcinomas: lessons learned from a 10-year experience. Korean J Radiol. 2008. 9:268–274.

5. Seki S, Sakaguchi H, Hagihara A, Fujii H, Kobayashi S, Iwai S, et al. Transcatheter arterial chemoembolization for superficial hepatocellular carcinoma induces adhesion. Adv Med Sci. 2007. 52:66–70.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download