Abstract
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy of the elderly and immunocompromised patients. It is occasionally found coexisting with other diseases, such as squamous cell carcinoma, basal cell carcinoma, actinic keratosis, miscellaneous adnexal tumors, and rarely Bowen disease. A 75-year-old woman presented with a 6-month history of an irregularly shaped erythematous patch on the left mandibular angle. Three months later, a 1.5×1.0 cm sized painless and rapidly growing erythematous nodule developed on the patch. Microscopically, the patch lesion was consistent with that of Bowen disease. The nodular lesion showed a number of small uniform hyperchromatic cells with scanty cytoplasm. It showed dense small-cell like nodular infiltration in the dermis. Immunohistochemical staining for cytokeratin 20 showed a positive result with a dot-like perinuclear pattern. Additionally, the result for thyroid transcription factor-1 was negative, which is positive in small cell neuroendocrine carcinoma. From these findings, we diagnosed this lesion as MCC concurrent with Bowen disease.
Merkel cell carcinoma (MCC), also referred to as cutaneous neuroendocrine carcinoma, is an uncommon malignancy that has a predilection for locoregional recurrence and distal metastasis1. It is characterized by a painless, red-purple colored nodule or an indurated plaque on a sun-exposed area. It usually arises in the head and neck of elderly people and tends to be more common in men1. Although it is usually found as a solitary lesion, it is occasionally found to coexist with other malignancies, such as squamous cell carcinoma, basal cell carcinoma, and rarely Bowen disease2. Herein, we report an interesting case of MCC concurrent with Bowen disease.
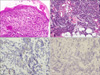
A 75-year-old woman presented with a 6-month history of an irregularly shaped erythematous patch on the left mandibular angle (Fig. 1). Three months later, a 1.5×1.0 cm sized painless and rapidly growing erythematous nodule developed on the patch. Her medical history and family history were unremarkable, and there was no history of chronic trauma to the left cheek. Laboratory findings, including complete blood count, blood chemistry, and routine urinalysis, were normal. The skin biopsy specimens were taken from the surrounding erythematous patch and the nodular lesion, respectively. Histopathologically, the patch lesion showed full-thickness keratinocytic atypia resulting in a "windblown appearance" of the epidermis, consistent with Bowen disease (Fig. 2a). The nodular lesion showed numerous small uniform cells with round hyperchromatic nuclei and scanty cytoplasms (Fig. 2b). We regarded this lesion as a malignant lymphoma and performed special stains. However, stain results for these cells were negative to pan T cell (CD3) and pan B cell (CD79a and CD20) markers. The immunohistochemical stain results for chromogranin and synaptophysin were focally positive, and the result for cytokeratin 20 (CK20) was positive with a dot-like perinuclear pattern (Fig. 2c). Additionally, the stain result for thyroid transcription factor-1 (TTF-1) to perform the differential diagnosis between MCC and small cell lung cancer was negative (Fig. 2d). From these findings, we diagnosed this lesion as a MCC concurrent with Bowen disease. The evaluations for systemic involvement and surgical treatment were recommended. Consequently, she was transferred to the plastic surgery department, but she just received a palliative treatment.
MCC is a primary cutaneous carcinoma, which is a rare malignant tumor that was described first as a trabecular carcinoma of the skin by Toker3 in 1972. Although the exact origin of the MCC is unknown, Tang and Toker4 suggested in 1978 that tumor cells may arise from Merkel cells because the cells contain electron-dense core granules which are believed to be a feature of Merkel cells.
Histopathologically, the epidermis is involved in less than 10% of all MCC cases, and the tumor cells are located in the dermis and grow toward subcutaneous tissue, with a tendency to infiltrate vascular and lymphatic vessels1,2. The tumor cells are uniformly sized basophilic cells, with round or oval nuclei and small nucleoli. Immunohistochemical stains are useful for differential diagnosis between MCC and other cutaneous tumors. CK20, which is the most widely used and the single most useful immunohistochemical stain in the work-up of MCC, is positive; its perinuclear dot-like expression is the hallmark staining pattern in MCC. Additionally, the tumor cells stain positively for neuron-specific enolase, chromogranin, synaptophysin, and neurofilaments. Staining for S100 protein, TTF-1, glial fibrillary acidic protein, actin, vimentin, and leukocyte common antigen1 is negative. In our case, the tumor cells were negative for pan T cell (CD3) and pan B cell (CD79a and CD20) markers, positive for CK20, and negative for TTF-1.
MCC is usually found as a solitary lesion but is occasionally found to coexist with other diseases, such as squamous cell carcinoma, basal cell carcinoma, actinic keratosis, miscellaneous adnexal tumors, and rarely Bowen disease (Table 1)1,2,5-9. Two theories are postulated to explain these observations. Smith et al.10 suggested that MCC may arise from a primitive pluripotent stem cell that has the capacity to differentiate along different cell lines. They reviewed 132 MCCs and found 11 cases with an intraepidermal component and focal squamous and/or eccrine differentiation. On the other hand, Gomez et al.11 suggested that there may be a possible common carcinogenic influence on different precursor cells of the skin that cause the coexistence of different tumors. They described 11 cases of MCC associated with invasive squamous cell carcinoma in the same area. When the neoplasms were coexisting, there was never a suggestion of continuity between them, and transitional cell forms were not seen.
The exact cause of why both MCC and Bowen disease can exist concurrently is still unknown to us. It has been reported that some cases of MCC are concurrent with Bowen disease occurring in the same region synchronously2,5-9. Several studies suggested that arsenic is a carcinogen that may induce the occurrence of MCC in addition to squamous cell carcinoma, basal cell carcinoma, and Bowen disease7,8,12. In the past, it was common to take pills which included arsenic. Our patient did not remember whether she took pills in the past due to her old age. So we tried to get information from her family, but we could not find any proof of arsenic exposure during her history. Considering these facts, we can suppose that MCC and Bowen disease occured incidentally to our patient.
The preferred treatment at present is surgical excision with sentinel lymph node biopsy followed by lymph node dissection if the latter is positive. Postoperative radiotherapy is also given, and this approach improves locoregional control, in addition to disease-free survival. Chemotherapy is not routinely recommended, as it does not increase overall survival1,8.
We clinically observed a case of the MCC concurrent with Bowen disease occurring at a sun exposure location and without any known risk factors. It is a rare case in the dermatologic literature; thus, physicians should consider that MCC could be included in the differential diagnosis of nodular lesions in Bowen disease.
Figures and Tables
Fig. 1
The erythematous nodule based on an irregular shaped erythematous patch is seen on the left mandibular angle.
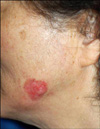
Fig. 2
(a) "Windblown appearance" of the epidermis on the patch lesion (H&E, ×200). (b) Numerous small uniform tumor cells with round hyperchromatic nuclei and scanty cytoplasm are arranged nodular infiltration in the dermis on the nodular lesion (H&E, ×100). (c) Cytokeratin 20 was stained positive in a dot-like perinuclear pattern (×200). (d) Immunohistochemical staining of tumor cells showing negativity for thyroid transcription factor 1 (×200).
References
1. Nghiem P, Jaimes N. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Merkel cell carcinoma. Dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1087–1094.
2. Sirikanjanapong S, Melamed J, Patel RR. Intraepidermal and dermal Merkel cell carcinoma with squamous cell carcinoma in situ: a case report with review of literature. J Cutan Pathol. 2010. 37:881–885.

4. Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978. 42:2311–2321.

5. Schenk P, Konrad K. Merkel cell carcinoma of the head and neck associated with Bowen's disease. Eur Arch Otorhinolaryngol. 1991. 248:436–441.

6. Okamoto O, Yoshiyama M, Takayasu S, Yokoyama S. Merkel cell carcinoma: report of three cases. J Dermatol. 1998. 25:45–50.

7. Tsuruta D, Hamada T, Mochida K, Nakagawa K, Kobayashi H, Ishii M. Merkel cell carcinoma, Bowen's disease and chronic occupational arsenic poisoning. Br J Dermatol. 1998. 139:291–294.

8. Moon YJ, Yi JH, Choi HS, Yun SK, Kim HU, Ihm CW. A case of merkel cell carcinoma in association with bowens disease. Korean J Dermatol. 2004. 42:309–312.
9. Sarma DP, Wang B, Shehan J, Linder-Stephenson L. Concurrent Merkel cell carcinoma and Bowen's disease of the thigh. Int J Dermatol. 2007. 5.

10. Smith KJ, Skelton HG 3rd, Holland TT, Morgan AM, Lupton GP. Neuroendocrine (Merkel cell) carcinoma with an intraepidermal component. Am J Dermatopathol. 1993. 15:528–533.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download