Abstract
There have been no long-term complications or life-threatening adverse effects related to botulinum toxin treatment for any cosmetic indications. Nevertheless, there are well-known, mild side effects of botulinum toxin treatment on the upper face, though most of them are self limited with time. However, excluding brow ptosis, reports about site specific side effects are few and anecdotal. We experienced cases of exaggeration of wrinkles after botulinum toxin injection for forehead horizontal lines, and report them here. In our cases, new appearance of a noticeable glabellar protrusion following botulinum toxin injection on the forehead was observed in 2 patients. Also, a new deep wrinkle on one side of the forehead just above the eyebrow appeared in another 2 patients. The exaggerated wrinkles nearly disappeared without treatment by week 4 in all subjects. These exaggerations of wrinkles may be caused by hyperactivity and overcompensation of untreated muscles. With the increasing availability of diverse botulinum toxin for cosmetic purposes, physicians and patients should be aware of this temporary change after therapeutic injections. We recommend explaining this possible effect prior to injection, for better understanding of treatment for cosmetic indications.
Botulinum toxin injection is widely used to treat a variety of hyperfunctional facial lines. The safety and efficacy of botulinum toxin for the treatment of upper facial rhytides, including the forehead, have been demonstrated in several studies1-3. When properly used, the incidence of complications with botulinum toxin is low, and there have been no long-term complications or life-threatening adverse effects related to botulinum toxin treatment for any cosmetic indications4. However, there are well-known, mild side effects of botulinum toxin treatment on the upper face, though most of them are self limited with time5. Common adverse effects can occur due to percutaneous injections on the forehead, and include pain, edema, erythema, and ecchymosis. Site specific side effects associated botulinum toxin injection for forehead horizontal lines include brow ptosis, exaggeration of wrinkles, and periorbital edema6. However, excluding brow ptosis, reports about site specific side effects are few and anecdotal. We experienced 4 cases of exaggeration of wrinkles after botulinum toxin injection for forehead horizontal lines, and these cases are reported here.
Our patients had mild or moderate forehead wrinkling prior to treatment, and were treated by less than 20 units of botulinum toxin (Botox®, Allergan, Irvine, CA, USA). Treatment was standardized to include five intramuscular injection sites (midpupillary line, halfway between the eyebrows and the hairline). The new appearance of a noticeable glabellar protrusion following botulinum toxin injection on the forehead was observed in 2 patients. In addition, a new deep wrinkle on one side of the forehead just above the eyebrow appeared in another 2 patients. The exaggerated wrinkles nearly disappeared without treatment by week 4 in all subjects. We report 4 cases of temporary exaggeration of wrinkles after botulinum toxin treatment.
In this case, a 41-year-old woman with moderate forehead wrinkling was treated by botulinum toxin. Decrease of upper forehead wrinkling with the appearance of lower frontalis and glabellar protrusion was shown one week after treatment (Fig. 1B, arrows). By four weeks, this protrusion had diminished and remained absent through 16 weeks follow-up (Fig. 1).
This 49-year-old woman had moderate forehead wrinkling at maximal upward gaze, prior to treatment. Two weeks after treatment, forehead wrinkling was significantly decreased. However, there was a new appearance of forehead lines that remained at week 4 (Fig. 3B, C, arrows) and showed marked reduction at week 8 (Fig. 3).
This 33-year-old woman had minimal forehead wrinkling prior to treatment. One week after treatment, there appeared to be a new deep wrinkle on the left side of her forehead above the eyebrow, that decreased by week 2 and disappeared completely by week 4 (Fig. 4).
The frontalis is a large, thin muscle closely attached to the skin. Its medial fibers are joined at the glabellar region, where they intersect with the procerus. Its central and lateral fibers blend in with the corrugator supercilii and the inner part of the orbicularis oculi. Contraction of the frontalis raises the eyebrows and the upper eyelid, causing the formation of horizontal forehead rhytides7. The lower 2.5 to 4.0 cm of the frontalis muscle moves cephalad to elevate the eyebrows8, and over time, repeated muscle contraction can result in the formation of rhytides.
Botulinum toxin can improve facial rhytides via weakness or paralysis of these muscles9,10. For the treatment of forehead horizontal lines, the injection points should always be 4~5 cm above the orbital rim. As a result, paralysis of the frontalis is limited to the upper part of the frontalis and compensatory hyperactivation of the lower part of frontalis muscle can be possible in the lateral part of forehead. Therefore, especially in people frequently using the frontalis muscle to raise their eyebrows or eyelids, exaggeration of previously unidentifiable wrinkles can take place at the border between the paralyzed frontalis muscle and non-paralyzed frontalis muscle. In the medial part of the forehead, the hyperactivation of glabellar muscles can be possible because of similar reasons. This hyperactivation of glabellar muscles with weakness of the frontalis muscle may cause the protruding of glabellar area in our cases.
In our cases, the exaggeration of forehead lines after botulinum toxin treatment improved spontaneously without any further treatment. For the compliance and satisfaction of patients, additional botulinum toxin injection at hyperactivated muscles can be performed. However, careful attention to the other side effects such as brow ptosis or eyelid ptosis should be warranted in additional injection.
Our cases demonstrated the temporary appearance of new wrinkles after botulinum toxin treatment. These may be caused by hyperactivity and overcompensation of untreated muscles. With the increasing availability of diverse botulinum toxin for cosmetic purposes, physicians and patients alike should be aware of this temporary change after therapeutic injection. We recommend explaining this possible effect prior to injection, for better understanding of treatment for cosmetic indications. We found that exaggeration of wrinkles on untreated areas of the forehead are self limited, and botulinum toxin treatment in this area should ultimately result in a satisfying cosmetic outcome for the patient.
Figures and Tables
Fig. 1
Case 1: Patient before treatment at maximal forehead wrinkling (A), one week after treatment (B) and at 4 (C) and 16 (D) week follow-ups.
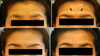
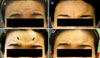
Fig. 2
Case 2: Forehead wrinkling at maximal upward gaze prior to treatment (A), and at 1, 2, and 4 weeks' follow-up after botulinum toxin treatment (B~D).
References
1. Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel- group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Dermatol Surg. 2003. 29:461–467.

2. Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002. 46:840–849.

3. Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, et al. One-year, randomized, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. J Clin Res. 2004. 7:1–20.
4. Hambleton P, Moore AP. Moore P, editor. Botulinum neurotoxins: origin, structure, molecular actions and antibodies. Handbook of botulinum toxin treatment. 1995. 1st ed. Oxford: Blackwell Science;16–27.
5. Rzany B, Dill-Müller D, Grablowitz D, Heckmann M, Caird D. Repeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4,103 treatments in 945 patients. Dermatol Surg. 2007. 33:S18–S25.

6. Youn SW, Seo KI, Yoo JY, Park KC, Eun HC. A clinical study of facial wrinkles affected by facial expression muscles treated with botulinum toxin (Botox®). Korean J Dermatol. 2002. 40:386–392.
7. Ascher B, Talarico S, Cassuto D, Escobar S, Hexsel D, Jaén P, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)--Part I: Upper facial wrinkles. J Eur Acad Dermatol Venereol. 2010. 24:1278–1284.

8. Klein AW. Complications, adverse reactions, and insights with the use of botulinum toxin. Dermatol Surg. 2003. 29:549–556.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download