Dear Editor:
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and fatal lung disease with inevitable loss of lung function. Pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone) is a novel synthetic molecule used for the treatment of IPF1. It inhibits the activity of inflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1, and increases the production of anti-inflammatory cytokine IL-101. Although pirfenidone is a promising drug for the treatment of IPF, adverse effects such as rashes and photosensitivity often occur1.
A 65-year-old man was referred to our dermatology department (Fig. 1) with scaly erythema of the face, hands, and forearms. He had been diagnosed with IPF in our pulmonology department seven months prior. One month after he was diagnosed, he was placed on pirfenidone (Pirespa®; Ildong Pharmaceutical Co., Seoul, Korea), with a gradual increase in dose until 1,200 mg/day was reached. He showed good tolerance for 4 months. The patient usually was outside for no more than 2 hours and always used sunscreen. However, two weeks before consultation, he had an unusually long sun exposure for more than 4 hours without using sunscreen. Two days after exposure, he noticed pruritus and erythema on his face and hands. A photosensitivity test for ultraviolet A (UV-A; operative wavelength of 320~400 nm), ultraviolet B (UV-B; 290~320 nm), and visible light rays was performed, and showed a markedly decreased minimum erythema dose (MED) of 28 mJ/cm2 with UV-B, and a positive response to UV-A, with a MED of 2 J/cm2. The patient discontinued pirfenidone and was started on oral cyclosporine 100 mg/day and oral hydroxychloroquine 400 mg/day. This treatment led to slow but complete resolution of the skin lesions within 6 weeks. Six weeks after the discontinuation of pirfenidone, irradiation with 15 J/cm2 UV-A induced no erythema and UV-B MED remained at 28 mJ/cm2 (Fig. 2). We advised the patient and pulmonologist to restart pirfenidone to avoid a setback in IPF. The patient restarted pirfenidone at a lower dose. He again slowly increased the dose and showed good tolerance without any recurrence for 2 months. We received the patient's consent form about publishing all photographic materials.
Pirfenidone is a newly developed antifibrotic synthetic molecule for the treatment of IPF1. Photosensitivity is an adverse effect of oral pirfenidone, with a frequency of about 50%2. The exact mechanism of pirfenidone-induced photosensitivity remains unclear. Seto et al.3 suggested that high doses of oral pirfenidone could cause phototoxic skin responses through a photoirritation pathway mediated by reactive oxygen species (ROS). Absorption of UV could result in skin lesions due to generation of ROS3.
In our case, the fact that the discontinuation of pirfenidone improved hypersensitivity to UV-A supported the diagnosis of pirfenidone-induced phototoxicity rather than photoallergy or photosensitive autoimmune disease45. However, because MED of UV-B remained at a reduced state, it is possible that other photosensitivity disorders may have been initiated by UV-B. The patient requires periodic evaluation of skin lesions.
In conclusion, we believe this is the first report of pirfenidone-induced photosensitivity in Korea. Clinicians should be aware of the potential phototoxic effects of oral pirfenidone. Patients on pirfenidone should be advised to avoid exposure to sunlight and should use photoprotective clothing and sunscreens.
Figures and Tables
Fig. 1
Multiple erythematous scaly patches and plaques on the anterior neck (A) and both forearms (B, C). Six weeks after the discontinuation of pirfenidone, multiple well demarcated hyperpigmented macules on the anterior neck (D) and both forearms (E, F).
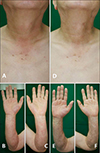
Fig. 2
A photosensitivity test showed a markedly decreased minimum erythema dose (MED) of 2 J/cm2 with ultraviolet A (UV-A) (A) and 28 mJ/cm2 with ultraviolet B (UV-B) (B). Six weeks after the discontinuation of pirfenidone, irradiation with 15 J/cm2 of UV-A induced no erythema (C) and UV-B MED remained at 28 mJ/cm2 (D).

References
1. Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011; 377:1760–1769.

2. Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010; 35:821–829.

3. Seto Y, Inoue R, Kato M, Yamada S, Onoue S. Photosafety assessments on pirfenidone: photochemical, photobiological, and pharmacokinetic characterization. J Photochem Photobiol B. 2013; 120:44–51.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download