Abstract
Elephantiasis is a symptom characterized by the thickening of the skin and underlying tissues in the legs. Pretibial myxedema (PTM) is a non-frequent manifestation of autoimmune thyroiditis, particularly Graves' disease. Lesions of myxedema occur most commonly on the pretibial surfaces, also develop at sites of previous injury or scars and other areas. A 49-year-old male presented with severe elephantiasis on the both pretibial areas, dorsum of the feet, ankles and toes. Twenty years previously, he had received radioactive iodine treatment for thyrotoxicosis. Laboratory tests showed that the patient's thyroid function was normal, but the level of thyroid stimulating hormone (TSH) receptor antibodies was very high (>40 IU/L). The biopsy confirmed PTM. Interestingly, the connective tissue was stained with the TSH receptor antibodies in the deep dermis. Elephantiasic PTM is a severe form of the myxedema and there is few reported case. We report a rare case of PTM with appearance of severe elephantiasis.
Elephantiasis is a symptom characterized by the thickening of the skin and underlying tissues after excessive swelling associated with lymph accumulation. It is most marked in the lower limbs but also affect the scrotum in males, breast and arms. The diseases that have this symptom include elephantiasis nostras, lymphatic filariasis, podoconiosis, proteus syndrome, pretibial myxedema (PTM)1. PTM also called thyroid dermopathy is a non-frequent manifestation of autoimmune thyroiditis, particularly Graves' disease. Lesions of myxedema occur most commonly on the pretibial surfaces, also develop at sites of the surgical scar, in area exposed to repetitive trauma, and after episodes of prolonged standing2. Interaction between thyroid stimulating hormone (TSH) receptor in skin fibroblasts and TSH receptor antibodies in the serum of patients with PTM is major role in the pathogenesis of dermopathy3. In most case, PTM is self-limited and mild, but advanced cases may cause cosmetic or functional problems4. We herein report a case of 49-old-year man with PTM presenting as elephantiasis.
A 49-year-old male presented with progressive bilateral lower limb swelling with thickening and induration of the skin over a span of 20 years. The physical examination revealed severe elephantiasis that was multiple violaceous polypoid, verrucous nodules and cerebriform hypertrophic plaques on the both pretibial areas, dorsum of the feet, ankles and toes (Fig. 1). Bilateral exophthalmos, clubbed fingers were seen, but no thyromegaly. Twenty years previously, he had received radioactive iodine treatment for thyrotoxicosis. He recalled that there was no trauma history. He had a 10 pack-year history of smoking and have quit in the past 5 years. Laboratory tests showed that the triiodothyronine (T3), thyroxine (T4), TSH was normal, but the level of TSH receptor antibodies was very high (>40 IU/L). We conducted polymerase chain reaction test and antibody test to rule out the possibility of filarial infection. The result was negative on both. Histopathologic findings revealed epidermal hyperkeratosis and collagen bundles were widely separated and fragmented with extensive deposition of mucin in the entire dermis (Fig. 2A). Alcian blue stain confirmed abundant deposition of mucin in the dermis (Fig. 2B). Interestingly, the connective tissue was stained with the TSH receptor-antibodies, probably in dermal fibroblasts, in the deep dermis (Fig. 2C). Based on these findings, he was diagnosed with PTM. We developed a treatment plan. First, he had submitted to an operation to remove massive proliferations of fibrous connective tissue and then intended to have topical and intralesional corticosteroid therapy to prevent recurrence following surgery. So, he referred to orthopedics for having a debulking surgery. Under anaesthesia, the tumours of left lower extremity were removed and the overlying epidermis prepared to receive meshed split-thickness skin grafts. After the operation, he received intralesional injection of 10 mg triamcinolone acetonide once and then referred to orthopedics to undergo further surgery. At 9-month follow-up there was no recurrence (Fig. 3). We received the patient's consent form about publishing all photographic materials.
PTM or thyroid dermopathy is a known manifestation of Graves' disease and characterized by bilateral, asymmetric, nonpitting scaly thickening of the skin limited to the pretibial area. PTM most commonly develops in old age adults, peak age at onset in the fifth to sixth decades of life. PTM occurs in 0.5 % to 4.3% of patients with a history of thyrotoxicosis of Graves' disease, but small number of patients may present without hyperthyroidism and some may be hypothyroid or euthyroid. Generally thyrotoxicosis develops first, followed by ophthalmopathy and dermopathy appear later in the course of disease4.
The pathogenesis of PTM is multifactorial3. First, TSH receptor antibodies in the serum of patients with PTM act directly on skin fibroblasts by stimulating the synthesis of glycosaminglycans (GAGs), which are major constituents of mucin. These patients have very high serum concentration of TSH receptor antibodies and overexpressed TSH receptors on fibroblasts induced by certain cytokines or local factors such as interleukin-6 (IL-6) in pretibial tissues5. Recent studies have proposed that insulin-like growth factor-1 (IGF-1) receptor on fibroblasts interacts with Graves' disease immunoglobulins to cause upregulation of T-cell chemoattractants such as IL-16, regulated on activation, normal T-cell expressed, and secreted (RANTES) and activation of fibroblasts6. Second, activation of fibroblasts could be indirect through sensitized T cells. Infiltrated thyroid-specific T cells release cytokines, including IL-1α and TGF-β, stimulating the synthesis of GAGs by fibroblasts. Third, trauma or injury may lead to activation of T cells and initiation of an antigen-specific response, which result in stimulation of fibroblasts to produce GAGs2. Fourth, tobacco use also is risk factor for development of PTM. Locally increased GAGs lead to the accumulation of fluid and the expansion of dermal connective tissue to cause compression of dermal lymphatics, resulting in clinical presentations seen in lymphedema.
PTM is classified into one of the following four forms: diffuse non-pitting edema, plaque, nodular and elephantiasic form. The elephantiasic form is the most severe, symptomatic form, which occurs in 5% of patients and may be quite resistant to any treatment4. The treatment of elephantiasic PTM is rarely documented and there are no standard treatment guidelines. Various treatments for elephantiasic PTM include topical, intralesional, and systemic steroids; compression therapy; radiotherapy; plasmapheresis alone or in combination with immunosuppressive agents; intravenous immunoglobulin; surgical therapy (Table 1)789101112131415.
In a study by Schwartz et al.4 they had 47.9% partial or complete remission rate among more severe cases treated with corticosteroids compared with a 55.6% partial or complete remission rate among patients with milder disease who did not receive any treatments. Therefore, they recommend that patients with more severe dermopathy are more likely to receive local treatments, but patients with mild disease are more likely to receive no treatment at all. Local corticosteroid therapy is commonly recommended for mild and severe cases. There were several reports that skin lesions were improved by the directly topical application of glucocorticoid to the lesion with hydrocolloid or a plastic wrap occlusive dressing during 4~10 weeks3. But, severe case may persist despite local corticosteroid therapy and even very severe elephantiasic PTM may more refractory to aggressive local corticosteroid therapy or systemic immune modulation4.
In our case, immunohistochemistry revealed that the connective tissue was mildly stained with TSH receptor antibodies, probably in dermal fibroblasts, in the deep dermis. We suspect that PTM was developed by the interaction TSH receptor antibodies in the serum of patient and TSH receptors on dermal fibroblasts and tobacco use may be a risk factor for the aggravation of dermopathy. Because the lesion was so severe in our case, which created mechanical and functional disability of patient, it was necessary to treat his skin lesions. Although trauma or surgery scar is associated with development of myxedema, debulking surgery combined with local corticosteroid therapy is recommended for treatment of severe elephantiasic PTM. Surgical excision is a rapid, effective method to remove massive fibrous connective tissue and then local corticosteroid therapy prevents recurrence of PTM following surgery. Lan et al.14 a 43-year-old male patient with severe elephantiasic PTM had a surgery and then were treated with intralesional injection of triamcinolone acetonide and there was no recurrence at 1 year follow-up. Felton et al.15 surgery combined with intralesional octreotide injection was an effective treatment to elephantiasic PTM and reported that there was no recurrence during 9 years.
In conclusion, we found the increase of TSH receptor expression on fibroblast through TSH receptor antibody staining and there are no published paper reporting this profile of elephantiasic PTM. There are several reported cases of elephantiasic pretibial myxedema. However, to date, there are no published cases of mass like skin lesion, invading all the way to the ankle, heel, and toe like this case. Also, because this case is a very severe form of PTM, it may be ineffective to treat with local corticosteroid therapy alone. So the treatment of choice to suitable for this case is a debulking surgery followed by local corticosteroid therapy. We report a rare case of PTM with appearance of severe elephantiasis.
References
1. Yimer M, Hailu T, Mulu W, Abera B. Epidemiology of elephantiasis with special emphasis on podoconiosis in Ethiopia: a literature review. J Vector Borne Dis. 2015; 52:111–115. PMID: 26119541.
2. Davies TF. Trauma and pressure explain the clinical presentation of the Graves' disease triad. Thyroid. 2000; 10:629–630. PMID: 11014305.

3. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005; 6:295–309. PMID: 16252929.
4. Schwartz KM, Fatourechi V, Ahmed DD. Dermopathy of graves' disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002; 87:438–446. PMID: 11836263.

5. Rapoport B, Alsabeh R, Aftergood D, McLachlan SM. Elephantiasic pretibial myxedema: insight into and a hypothesis regarding the pathogenesis of the extrathyroidal manifestations of Graves' disease. Thyroid. 2000; 10:685–692. PMID: 11014313.

6. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003; 170:6348–6354. PMID: 12794168.

7. di Meo N, Nan K, Noal C, Trevisini S, Fadel M, Damiani G, et al. Polypoid and fungating form of elephantiasic pretibial myxedema with involvement of the hands. Int J Dermatol. 2016; 55:e413–e415. PMID: 26873132.

8. Shirai K, Ito T, Mitsuhashi Y, Tsuboi R. Dramatic effect of low-dose oral steroid on elephantiasic pretibial myxedema. J Dermatol. 2014; 41:941–942. PMID: 25200964.

9. Yu H, Jiang X, Pan M, Huang R. Elephantiasic pretibial myxedema in a patient with graves disease that resolved after 131I treatment. Clin Nucl Med. 2014; 39:758–759. PMID: 24830877.

10. Heyes C, Nolan R, Leahy M, Gebauer K. Treatment-resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012; 53:e1–e4. PMID: 22309343.

11. Dhaille F, Dadban A, Meziane L, Fessier C, Colta L, Lok C, et al. Elephantiasic pretibial myxoedema with upper-limb involvement, treated with low-dose intravenous immunoglobulins. Clin Exp Dermatol. 2012; 37:307–308. PMID: 22007759.

12. Terheyden P, Kahaly GJ, Zillikens D, Bröcker EB. Lack of response of elephantiasic pretibia myxoedema to treatment with high-dose intravenous immunoglobulin. Clin Exp Dermatol. 2003; 28:224–226. PMID: 12653721.
13. Susser WS, Heermans AG, Chapman MS, Baughman RD. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002; 46:723–726. PMID: 12004314.

14. Lan C, Li C, Yang M, Mei X, He Z, Chen W, et al. Pretibial myxoedema with autoimmunity and hyperplasia treated with glucocorticoids and surgery. Br J Dermatol. 2012; 166:457–459. PMID: 21848686.

15. Felton J, Derrick EK, Price ML. Successful combined surgical and octreotide treatment of severe pretibial myxedema reviewed after 9 years. Br J Dermatol. 2003; 148:825–826. PMID: 12752151.
Fig. 1
Multiple violaceous polypoid, verrucous nodules, cerebriform hypertrophic plaques and orange peel appearance on the both pretibial areas, dorsum of the feet, toes and ankles.
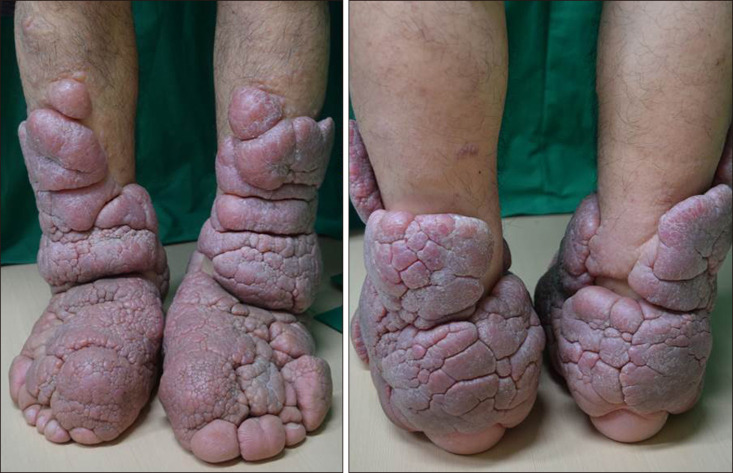
Fig. 2
(A) Fragmentation and fraying of collagen fibers and large depositions of mucin in the dermis. (B) Abundant deposition of mucin in the dermis. (C) In the deep dermis, the connective tissue was stained with the thyroid stimulating hormone (TSH) receptor-antibodies, probably in dermal fibroblasts (A: H&E, ×40; B: Alcian blue, ×40; C: TSH receptor antibody, ×100).
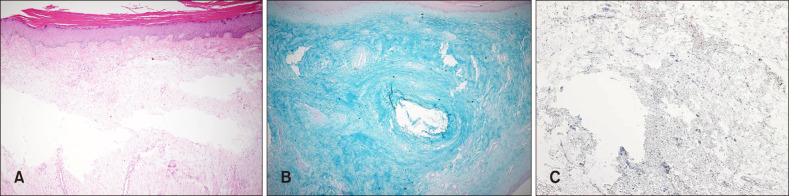
Fig. 3
At 9 months of follow-up, erythema with dark brownish colored pigmentation and plaques on the shins and dorsa of the feet, but note significantly decreased edema, nodularity.

Table 1
Reported treatment of elephantiasic pretibial myxedema
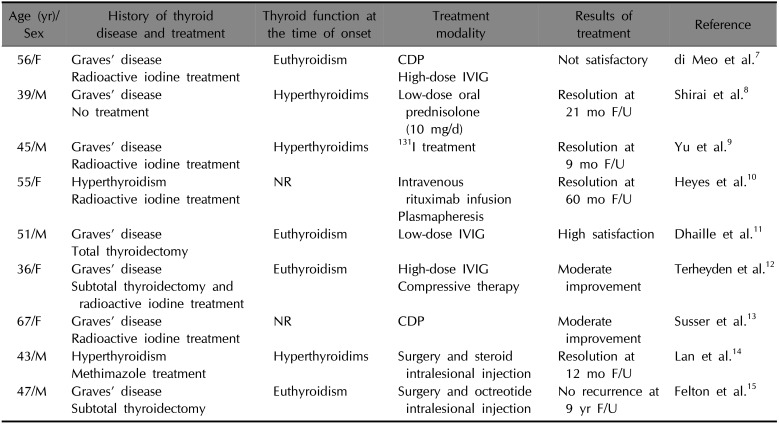
| Age (yr)/Sex | History of thyroid disease and treatment | Thyroid function at the time of onset | Treatment modality | Results of treatment | Reference |
|---|---|---|---|---|---|
| 56/F | Graves' disease | Euthyroidism | CDP | Not satisfactory | di Meo et al.7 |
| Radioactive iodine treatment | High-dose IVIG | ||||
| 39/M | Graves' disease | Hyperthyroidims | Low-dose oral prednisolone (10 mg/d) | Resolution at 21 mo F/U | Shirai et al.8 |
| No treatment | |||||
| 45/M | Graves' disease | Hyperthyroidims | 131I treatment | Resolution at 9 mo F/U | Yu et al.9 |
| Radioactive iodine treatment | |||||
| 55/F | Hyperthyroidism | NR | Intravenous rituximab infusion | Resolution at 60 mo F/U | Heyes et al.10 |
| Radioactive iodine treatment | |||||
| Plasmapheresis | |||||
| 51/M | Graves' disease | Euthyroidism | Low-dose IVIG | High satisfaction | Dhaille et al.11 |
| Total thyroidectomy | |||||
| 36/F | Graves' disease | Euthyroidism | High-dose IVIG | Moderate improvement | Terheyden et al.12 |
| Subtotal thyroidectomy and radioactive iodine treatment | Compressive therapy | ||||
| 67/F | Graves' disease | NR | CDP | Moderate improvement | Susser et al.13 |
| Radioactive iodine treatment | |||||
| 43/M | Hyperthyroidism | Hyperthyroidims | Surgery and steroid intralesional injection | Resolution at 12 mo F/U | Lan et al.14 |
| Methimazole treatment | |||||
| 47/M | Graves' disease | Euthyroidism | Surgery and octreotide intralesional injection | No recurrence at 9 yr F/U | Felton et al.15 |
| Subtotal thyroidectomy |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download