Abstract
Background
Cellulite is a 'cottage cheese-like' cutaneous change caused by subcutaneous fat bulging into the dermis that usually leads to cosmetic problems. Slimming cream containing 3.5% water-soluble caffeine and xanthenes exhibits a lipolytic effect with penetration into the dermis.
Methods
Fifteen subjects with cellulite applied slimming cream to the thighs and inner side of the upper arms twice daily for 6 weeks. Efficacy was assessed using a standard visual scale, changes in the circumferences of the thighs and upper arms, and patient satisfaction by a questionnaire at baseline, week 3, and week 6. Safety was assessed by inquiring about adverse events through questionnaires.
Results
The standard visual scale score improved significantly by 0.49 points (19.8%) at week 6. Thigh and upper-arm circumferences decreased by 0.7 cm (1.7%) and 0.8 cm (2.3%), respectively, at week 6. Slight itching and transient flushing were commonly reported, but no serious adverse event occurred.
Cellulite is the dimpling of skin surface that exhibits an 'orange peel-like' or 'cottage cheese-like' appearance; it mostly occurs in the gluteal-femoral region of post-adolescent women. It approximately affects in 85% women older than 20 years1. Subcutaneous fat protrusion into the dermis is considered to be the major cause, but its mechanism is not clearly understood2,3. Cellulite is caused by several factors including enlarged fat lobules, excessive tension, stress, or decreased collagen4,5,6. Cellulite usually leads to cosmetic problems, but its treatment is not well established.
Slimming cream (ZONE·5 Slimming Zone Smart Silhouette Cream; Skin & Tech, Seongnam, Korea) contains 3.5% water-soluble caffeine and xanthenes, and exerts lipolytic effects through the inhibition of phosphodiesterase (PDE) and induction of cyclic adenosine monophosphate (cAMP) in adipocytes7. Caffeine can penetrate the skin barrier to reach the dermis where fat protrusion occurs. Commercially available slimming cream contains 3% caffeine8. Although caffeine is basically hydrophilic, its water solubility is very low. Therefore, higher-concentration formulas require large amounts of surfactant or alcohol, which can cause skin irritation. The slimming cream tested in the present study contains water-soluble caffeine invented by the manufacturer;this cream contains 3.5% caffeine, which is a higher concentration than other commercially available forms with only a small amount of surfactant. Therefore, it is expected to provide better clinical efficacy without increased skin irritation. Accordingly, this study evaluated the efficacy and safety of the abovementioned slimming cream for the treatment of cellulite.
A total of 15 healthy female volunteers with cellulite on the thigh and medial side of the upper arms were enrolled. Subjects who were using drugs to treat cellulite or any agents that could affect fat metabolism within the last 6 months were excluded. Subjects who had a skin disease on the thighs or upper arms except cellulite, or were pregnant or breastfeeding were also excluded. This study was approved by the Institutional Review board of Seoul National University Bundang Hospital (IRB approval number: B-1212/181-002), and written informed consent was obtained from all subjects prior to participation.
Subjects applied slimming cream (ZONE·5 Slimming Zone Smart Silhouette Cream) containing 3.5% water-soluble caffeine and xanthenes on the thighs and upper arms twice daily for 6 weeks. The slimming cream includes water-soluble caffeine (i.e., caffeine and niacinamide, and no harmful organic solvent or surfactant), Slimexir (i.e., levan, caffeine, xanthenes, and decyl glucoside; RAHN Cosmetics Co., Zurich, Switzerland), coffee extract, Ilex paraguariensis leaf extract, green tea extract, Garcinia cambogia fruit extract, jojoba esters, and soybean oil.
Body weight was measured at every visit. Subjects dropped out their body weight changed more than 2 kg. Data were analyzed to assess mean changes among baseline, week 3, and week 6.
Efficacy assessments were performed at baseline, week 3, and week 6 and included a standard visual scale, measurement of thigh and upper-arm circumference, and a questionnaire. The standard visual scale proposed by Bielfeldt et al.9 was used to assess cellulite (Fig. 1). Three independent investigators graded the severity of cellulite on the posterior thighs from 0 to 9, with larger numbers indicating more severe cellulite.
The most proximal site of the thighs and upper arms were marked with dots using a gentian violet surgical pen. Thigh and upper-arm circumference were measured at the sites of the gentian violet pen marks. Subjects answered questionnaires concerning the subjective improvement of cellulite, degree of moisture, elasticity, general satisfaction with the treatment, and the presence of skin irritation.
The degrees of moisture and elasticity were objectively measured on the thigh and medial side of the upper arm by a corneometer and cutometer, respectively, at every visit. The corneometer measures the degree of moisture as very dry (<35), dry (35~50), or sufficiently moisturized (>50). Cutometer values consist of R0 to R9 values. According to the principle, higher R2 or R7 values indicate increased skin elasticity and a lower R9 value indicates a decreased skin-tiring effect.
Safety was assessed by inquiring about local irritation or other adverse events through questionnaires.
Results including the visual grade of cellulite, thigh and upper-arm circumference, and corneometer and cutometer values were analyzed by the Friedman test. Null hypotheses of no difference were rejected at p<0.05. Statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
Fourteen subjects completed the trial, while 1 was excluded during follow-up. The median age was 38.8 years (range, 25~51 years). One subject was excluded from the analysis because of weight loss of 5 kg.
Mean body weight at baseline, week 3, and week 6 was 63.49, 63.71, and 63.71 kg, respectively (p>0.05) (Fig. 2). Cellulite according to the standard visual scale graded by 3 independent investigators improved significantly over 6 weeks (Fig. 3). Compared to baseline, the mean score decreased 0.49 points (19.8%) at week 6 (p<0.05). The mean circumferences of the thigh and most proximal site of the upper arm also decreased significantly by 0.7 cm (1.7%) and 0.8 cm (2.3%), respectively, at week 6 compared to those at baseline (Fig. 4).
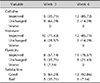
Table 1 presents the results of questionnaires regarding the subjective improvement of cellulite, degree of moisture, elasticity, and general treatment satisfaction. The results show that 5 of 14 subjects (35.71%) and 12 of 14 subjects (85.72%) reported improvement in cellulite at weeks 3 and 6, respectively. No aggravation of cellulite was reported during the trial. Moreover, 10 of 14 subjects (71.43%) and 12 of 14 subjects (85.71%) reported improvement in moisture, and 8 of 14 subjects (57.14%) and 11 of 14 subjects (78.57%) reported increased elasticity at weeks 3 and 6, respectively. Regarding satisfaction, 9 of 14 subjects (64.29%) and 13 of 14 subjects (92.86%) were generally satisfied with the product at weeks 3 and 6, respectively.
Skin irritation occurred in 8 of 14 subejcts (57.14%) at week 3 (Table 2). Transient flushing (21.43%) or slight itching (21.43%) were the most common, followed by erythema (7.14%) and prickling sensation (7.14%). At week 6, only 5 of 14 subjects (35.71%) complained of irritation; transient flushing (21.43%) was still the most common, followed by erythema (7.14%) and slight itching (7.14%).
The corneometer and cutometer measurements of the thigh and medial side of the upper arm are shown in Fig. 5. Moisture improved markedly. Meanwhile, skin elasticity improved according to increased R2 and R7 values and decreased R9 value, but the differences were not significant.
Cellulite usually causes cosmetic problems, but its treatment is not well established. Because the mechanism underlying cellulite is complex, combinations of treatments targeting different components are recommended. Xanthenes, caffeine, herbal extracts, retinoid, and peroxisome proliferator-activated receptors are administered topically to treat cellulite by reducing adipogenesis while inducing thermogenesis, microcirculation, and collagen synthesis7. Oral agents for the treatment of cellulite mainly aim to reduce weight, which is reported to improve cellulite severity; histologically, weight loss results in the retraction of fat globules out of the dermis10. Moreover, massage is also used to remove interstitial fluid and accelerate lymphatic drainage, which leads to the reduction of cellulite11.
Caffeine and xanthenes exert lipolytic effects via induction of cAMP and inhibition of PDE in adipocytes7. Elevated cAMP levels stimulate protein kinase A to phosphorylate and thus activate hormone-sensitive lipase. Phosphorylated hormone-sensitive lipase hydrolyzes triglycerides into diglycerides, monoglycerides, free fatty acids, and glycerol12. As the PDE enzyme decreases cAMP levels, the inhibition of PDE further increases cAMP activity13.
Clinical trials of topical agents containing caffeine or xanthenes for the treatment of cellulite have been performed owing to the lipolytic effects of these chemicals. Buscaglia and Conte14 report that combined use of caffeine, horse chestnut, ivy, algae, bladderwrack, plankton, butcher broom, and soy protein for 30 days reduced subcutaneous fat by 2.8 mm. A combination of topical caffeine-containing cream with inhalation of pepper, estragon, fennel, and grapefruit oils aiming to increase sympathetic neural activity is also reported to have a slimming effect15. On the other hand, xanthenes alone are reported to be ineffective for the treatment of cellulite; only 10% of subjects demonstrated improvement in a 12-week trial of topical aminophylline, an affiliate of methylxanthenes16. Differences in skin penetration might lead to the different efficacies. Because of its hydrophilicity, caffeine can penetrate the skin barrier and reach the dermis17,18. Interestingly, the penetration of caffeine is not reduced by increased skin thickness19.
The slimming cream tested in this study is the first topical agent containing both caffeine and xanthenes as well as other effective ingredients such as levan, decyl glucoside, I. paraguariensis leaf extract, etc. In this cream, caffeine is considered to play the major role in lipolysis by penetrating the dermis, while xanthenes have a synergistic effect. The subjects of the present study showed significant improvements in cellulite according to visual grade as well as thigh and upper-arm circumference. Patient satisfaction according to questionnaires was also high. These are very encouraging results for the development of new agents for the treatment of cellulite. Transient flushing and slight itching were common, occurring in 57.14% and 35.71% of the subjects at weeks 3 and 6, respectively. However, no adverse events led to treatment interruption or decreased cream use. No serious adverse event occurred.
Various devices for the treatment of cellulite have been studied. Alster and Tanzi20 report that 8 biweekly treatments with a device combining radiofrequency, infrared light, and mechanical tissue manipulation improved cellulite by approximately 50% and reduced thigh circumference by 0.8 cm. Meanwhile, Nootheti et al.21 report that a combination of low-energy diode laser, suction, and massage reduced upper-thigh circumference by 0.17 cm. A single treatment of 1,440-nm pulsed Nd:YAG laser showed good to excellent improvement of cellulite and a 30% increase in skin elasticity22. It is difficult to directly compare the efficacy of laser treatment with slimming cream because of differences in evaluation methods among studies. However, the effects slimming creams on thigh circumference are similar or superior to those of laser treatment. Furthermore, slimming creams appear to be safer, more inexpensive, and easier to use than laser treatment.
In cellulite, the elasticity of collagen in the dermis is an important factor that inhibits fat protrusion. As moisture degree also plays a role in skin strength, both elasticity and moisture degree were measured in the present study. Although most of the subjects reported improved moisture and elasticity in the questionnaire, the objectively measured values of moisture degree and elasticity showed no significant changes after treatment. Therefore, the association between dermal elasticity and cellulite requires further investigation.
In conclusion, the application of a slimming cream containing 3.5% water-soluble caffeine and xanthenes is effective and safe for the treatment of cellulite.
There are some limitations in this study. This was a simple single-center intervention study. The number of subjects was small (n=15). The long-term efficacy of the slimming cream tested was not evaluated. Therefore, additional clinical data are required to determine the long-term efficacy and safety of the slimming cream tested.
Figures and Tables
Fig. 1
Standard visual scale of cellulite grade. Data from the article of Bielfeldt, et al. Skin Res Technol 2008;14:336-3469.

Fig. 2
Mean body weight of subjects at baseline, week 3, and week 6. Body weight did not change significantly during the study (p>0.05).

Fig. 3
(A) Average visual grade of the cellulite rated by 3 independent investigators at baseline (V1), week 3 (V2), and week 6 (V3). The severity of cellulite improved significantly during the study. Visual grade was 0.49 points lower (19.8%) at week 6 than at baseline. (B) Clinical photographs of 2 representative cases at V1, V2, and V3. *p<0.05.
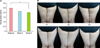
Fig. 4
Thigh and upper-arm circumferences at baseline, week 3, and week 6. The mean circumferences of the thigh and most proximal site of the upper arm decreased significantly during the study by 0.7 cm (1.7%) and 0.8 cm (2.3%) at week 6 vs. baseline. *p<0.05.

Fig. 5
Corneometer and cutometer measurements of the thigh and upper arm at baseline, week 3, and week 6. Moisture measured by corneometer and elasticity measured by cutometer did not change significantly in either the thigh or upper arm during 6 weeks (all p>0.05).

References
1. Cellulite meltdown? Harv Womens Health Watch. 1998; 5:7.
2. Querleux B, Cornillon C, Jolivet O, Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002; 8:118–124.

3. Piérard GE, Nizet JL, Piérard-Franchimont C. Cellulite: from standing fat herniation to hypodermal stretch marks. Am J Dermatopathol. 2000; 22:34–37.
4. Labat-Robert J. Age-dependent remodeling of connective tissue: role of fibronectin and laminin. Pathol Biol (Paris). 2003; 51:563–568.

5. Smalls LK, Hicks M, Passeretti D, Gersin K, Kitzmiller WJ, Bakhsh A, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006; 118:510–516.

6. Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004; 28:143–180.

8. Dias M, Farinha A, Faustino E, Hadgraft J, Pais J, Toscano C. Topical delivery of caffeine from some commercial formulations. Int J Pharm. 1999; 182:41–47.

9. Bielfeldt S, Buttgereit P, Brandt M, Springmann G, Wilhelm KP. Non-invasive evaluation techniques to quantify the efficacy of cosmetic anti-cellulite products. Skin Res Technol. 2008; 14:336–346.

10. Smalls LK, Lee CY, Whitestone J, Kitzmiller WJ, Wickett RR, Visscher MO. Quantitative model of cellulite: three-dimensional skin surface topography, biophysical characterization, and relationship to human perception. J Cosmet Sci. 2005; 56:105–120.

11. Draelos ZD, Marenus KD. Cellulite. Etiology and purported treatment. Dermatol Surg. 1997; 23:1177–1181.

12. Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol. 2007; 292:R77–R85.

13. Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008; 582:117–131.
14. Buscaglia DA, Conte ET. The treatment of cellulite with methylxanthine and herbal extract based cream: an ultrasonographic analysis. Cosmet Dermatol. 1996; 9:30–40.
15. Hariya T, Sakai K, Shibata Mea. Proposal of a novel slimming theory (UCP theory) and development of slimming odorants. In : Proceedings 6th Scientific Conference of the Asian Society of Cosmetic Scientists; 2003. p. 207–220.
16. Collis N, Elliot LA, Sharpe C, Sharpe DT. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999; 104:1110–1114. discussion 1115-1117.

17. Kim C, Shim J, Han S, Chang I. The skin-permeation-enhancing effect of phosphatidylcholine: caffeine as a model active ingredient. J Cosmet Sci. 2002; 53:363–374.
18. Trauer S, Patzelt A, Otberg N, Knorr F, Rozycki C, Balizs G, et al. Permeation of topically applied caffeine through human skin--a comparison of in vivo and in vitro data. Br J Clin Pharmacol. 2009; 68:181–186.

19. van de Sandt JJ, van Burgsteden JA, Cage S, Carmichael PL, Dick I, Kenyon S, et al. In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: a multi-centre comparison study. Regul Toxicol Pharmacol. 2004; 39:271–281.

20. Alster TS, Tanzi EL. Cellulite treatment using a novel combination radiofrequency, infrared light, and mechanical tissue manipulation device. J Cosmet Laser Ther. 2005; 7:81–85.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download