Abstract
Background
Lipid peroxide (LPO) in comedones, which are produced as a result of sebum oxidation, might potentially induce interleukin-1α (IL-1α) and exacerbate comedogenesis and inflammatory changes in comedones.
Objective
To investigate the relationship of proinflammatory cytokines and LPO levels in the extracts of comedones with the acne of clinical difference between smokers and non-smokers, and with the severity and distribution of the acne lesions.
Methods
Twenty-two non-smoking and 21 smoking adult acne patients were evaluated by comedone extraction and measurement of proinflammatory cytokines and LPO levels. Acne severity and distribution of the lesions were also analyzed.
Results
Relative to the non-smoking group, smokers had significantly higher levels of IL-1α and LPO in comedones. Their levels showed a positive correlation. However, there were no statistically significant difference between the severity or distribution of the disease and the levels of LPO and IL-1α in comedones.
Adult acne (postadolescent acne) can be defined as acne that presents in those over 25-years-of-age. It is classified into persistent and late-onset types, according to the age of onset1,2. Clinically, adult acne frequently involves the lower third of the face, chin, jaw line, and neck, and tends to manifest as mild to moderate inflammatory papules and pustules, with very few comedones2-4. However, thus far, no clear causes for the clinical difference between adult and adolescent acne have been delineated, although endocrine abnormality, genetic predisposition, antibiotic-resistant Propionibacterium acnes, cosmetics, smoking, and emotional stress are regarded as potential risk factors for the development of adult acne2,5. Among these risk factors, cigarette smoking and emotional stress inflict oxidative stress on the body6,7. Recent findings of significantly lower serum levels of antioxidant enzymes and antioxidants in acne patients prompted the postulation that oxidative stress be crucial in the pathogenesis of acne8.
The principal objective of this study was to clarify whether the levels of proinflammatory cytokines and lipid peroxide (LPO) in the extracts of comedones were significantly related to smoking, acne severity and distribution of acne lesions.
Informed consent was obtained from all patients and this study was approved by the Institutional Review Board of the Kyung Hee University Medical Center. A total of 50 patients with acne vulgaris (age 25 to 45 years, 23 males and 27 females) were enrolled in this study. A detailed smoking history was obtained during the first interview and patients were classified according to their smoking status as non-smokers, former smokers, and current smokers9. Non-smokers (n=22) were defined as those who had never smoked greater than 100 cigarettes or 5 packs of cigarettes in their lifetime. Current smokers (n=21) were defined as those who had smoked greater than 100 cigarettes in their lifetime and also had smoked in the 30 days preceding their interview. Former smokers (n=7) were defined as those who had smoked greater than 100 cigarettes in their lifetime, but had not smoked in the 30 days before their interview.
Patients were clinically evaluated to record the clinical severity of acne using the Korean acne grading system (KAGS), the duration of the disease, and the distribution of the lesions10. Patients who had received topical or systemic treatment over the previous 3 months were excluded. Patients were also excluded from the study if they had been diagnosed with acneiform eruption, rosacea, or demodex folliculitis.
Approximately 20 to 30 open comedones were aseptically collected from each patient using a comedone extractor after swabbing the skin surface with isopropanol. None of the samples were contaminated with blood. To prevent the oxidation of the collected samples, each sample was immediately placed in a lipid-free glass container, with the air replaced by nitrogen gas, and preserved at -80℃.
Total protein of each patient were extracted from the content of comedones by homogenization and ultrasound sonication (Sonicator XL2020; Misonix, Farmingdale, NY, USA) in 400 µl of 0.1% Triton in phosphate buffered saline. Insoluble materials were removed by a 5-minutes centrifugation at 16,000 g at 4℃ using a model 5402 microcentrifuge (Eppendorf, Hamburg, Germany). The supernatant was used for further experiments. Interieukin (IL)-1α, IL-6, and tumor necrosis factor-alpha (TNF-α) were measured using specific Quantikine® enzyme-linked immunosorbent assay kits (R&D systems, Minneapolis, MN, USA). Each specific optical density was measured with an E-ma® system (Molecular Devices, Sunnyvale, CA, USA). Data were expressed as ng/µg of total protein, which were measured with a BCA protein assay kit (Pierce, Rockford, IL, USA).
Comedone samples from each patient were homogenized and ultrasonicated in a solution containing equal quantities of acetone and diethyl ether, followed by lipid extraction. The extracted lipids were dried under reduced pressure and mixed with 1 ml of equal quantities of acetone and diethyl ether to measure the quantity of LPO and total lipids. LPO was quantified using a thiobarbituric acid (TBA) assay. The TBA solution consisting of 60 µl of 8% sodium dodecyl sulfate, 450 µl of 20% acetic acid (pH 3.5), 450 µl of 0.67% TBA, and 540 µl of MilliQ water was added to the dried samples and heated for 60 minutes at 95℃. To this reactive solution, 1.5 ml of n-butanol and pyridine solution at a ratio of 15 : 1 was added and centrifuged for 5 minutes at 640 g. The TBA value in the supernatant fluid was measured at an optical density of 532 nm using a UV-3101PC spectrophotometer (Shimadzu, Kyoto, Japan). Data are expressed as the TBA value per microgram of total lipids measured via the oxidation method, using chromium oxide calibrated with 0.05% palmitic acid/chloroform solution as a standard.
The Kruskal-Wallis test and Mann-Whitney test were employed to evaluate the differences in IL-1α and LPO levels among the groups divided by the smoking habit, KAGS, and lesion distribution. Pearson's correlation test was also employed to assess the relationships between LPO and IL-1α. Statistical significance was defined by a p-value<0.05. Statistical analyses were conducted using SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
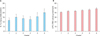
Smokers displayed significantly higher levels of both IL-1α and LPO in the comedones (p<0.05; Fig. 1). We did not include the statistical findings of IL-6 or TNF-α, since the level of each in the comedone contents proved undetectable in the majority of the patients, except for some with insignificant levels. Thus, statistical analyses could not be performed.
We compared IL-1α and LPO level between former (n=7) and current (n=21) smokers to assess the cumulative effect of smoking. There was no significant difference in IL-1α and LPO level between those two groups. IL-1α tended to be higher in current smokers (Fig. 2).
Five patients were KAGS grade 1, 8 patients were grade 2, 11 patients were grade 3, 11 patients were grade 4, 5 patients were grade 5, and 3 patients were grade 6. The quantities of IL-1α and LPO in the content of comedones were not significantly different in any KAGS group (Fig. 3).
We compared the extracted contents of comedones of adult acne patients from their forehead, cheek, and chin. Ten samples were collected from the forehead, 14 from the cheek, and 19 from chin area lesions. We attempted to determine whether the proinflammatory cytokines or LPO in the content of comedones was related in any way to the distribution of lesions. No statistically significant differences were evident (Fig. 4).
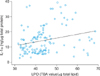
Levels of LPO and IL-1α were significantly positively correlated (r=0.251, p<0.05; Fig. 5).
The relationship of smoking and acne remains controversial. Capitanio et al.11 reported a positive correlation between cigarette smoking habits and adult women with acne. They also reported that cigarette smoking increases oxidative stress in sebum, which results in reductions in the level of the principal antioxidant, α-tocopherol, as well as increases in the levels of squalene peroxide in the sebum. This finding is consistent with our results. Thus, cigarette smoking can be regarded as a contributor to the initiation or exacerbation of adult acne.
Initially, we believed that we would find a significant difference between the level of IL-1α and acne severity in the content of comedones, since IL-1α is a well-known contributor to the initiation of inflammation in acne. Additionally, as adult acne usually involves the lower third of face and chin, we initially thought that we would observe some differences in the levels of LPO and IL-1α according to the distribution of the lesions. However, we found no statistically significant differences between the severity and distribution of the disease and the levels of LPO and IL-1α in the comedones. Our results are consistent with the study conducted by Arican et al.12, who reported no statistical difference or correlation between the severity and distribution of the acne and the levels of serum antioxidant enzymes and malondialdehyde (MDA), an end product of lipid peroxidation. Since this result may be due to the fact that our study employed only noninflamed comedones, further studies to assess the relationship between the severity and distribution of acne lesions and the status of oxidative stress will clearly be required.
The skin is constantly exposed to oxidative stresses induced by reactive oxygen species (ROS), which are generated both from endogenous sources such as enzyme activity or activated neutrophils, and from external stimuli such as non-ionizing radiation, cigarette smoking, stress, and even air pollution6,7,13. ROS-mediated oxidative damage can induce lipid peroxidation as well as DNA modification13.
Recently, it was demonstrated that systemic oxidative stresses may affect the pathogenesis of acne12. In patients with acne, the levels of antioxidant enzymes including catalase and glucose-6-phosphate dehydrogenase have been reported to be significantly lower than in normal controls, and the level of MDA was found to be statistically significantly higher12. It was also reported that the systemic levels of vitamins A and E were substantially lower overall in acne patients, and were negatively correlated with the degree of acne severity14. We believe it is possible that the antioxidants in our body are consumed at an accelerated rate in the processing of high-level oxidative stresses in acne patients relative to non-acne patients. Commonly used acne treatments, such as tetracycline, erythromycin, minocycline, metronidazole, and isoretinoin, significantly inhibit the production of neutrophil-derived ROS12,15. Therefore, antioxidant oral supplementation or topical application may also prove effective in managing acne. In fact, sodium ascorbyl phosphate, a strong antioxidant, was used at a concentration of 5% in the production of a lotion applied topically for 12 weeks in an open human study group, and was shown to significantly reduce lipid peroxidation and improve acne16. Based on these previous study results, oxidative stress exists in acne and may play an important role in its pathogenesis. Our study revealed smoking is positively related to oxidative stress level. Other oxidative stress-inducible factors, such as strong sunlight, prevalence of sleeping disturbance, and emotional stress, were compared between smokers and non-smokers group. No significant differences were detected between two groups (data not shown).
LPO is a peroxide generated in the presence of ROS by the oxidation of unsaturated fatty acids. The level of lipid peroxidation can be used as a marker for oxidative stress in cells17. Accumulated sebum in the infundibulum is susceptible to oxidation by ROS. When sebum oxidation occurs, LPO forms in the comedones and can increase the levels of cytokines and chemokines involved in cutaneous inflammation17. Recently, Tochio et al.18 reported that LPO in comedones may result in an increase in IL-1α expression via nuclear factor-kappa B and may also be involved in the progression of comedogenesis and inflammatory changes in comedones. Our finding of a positive correlation in the levels of LPO and IL-1α support this involvement. On the other hand, Ottaviani et al.19 demonstrated that squalene peroxide, which is the most comedogenic LPO, induces an inflammatory response in keratinocytes via lipoxygenase activation and an increase in IL-6 production. Our data did not reveal statistically significant IL-6 levels. This may be attributable to the difference in the methods employed to measure the IL-6 in vitro. Previous studies concerning the levels of IL-6 and TNF-α in comedones have also demonstrated statistically insignificant levels of each component. Further advancement in laboratory techniques measuring these cytokine levels in comedones will be necessary to reveal increases20,21.
In conclusion, our study explains the role of oxidative stress in smoking adult acne patients. Cigarette smoking can induce lipid peroxidation of sebum in comedones by inflicting oxidative stress, resulting in increases in the local level of IL-1α, which consequently induces abnormal follicular keratinization or inflammation.
Figures and Tables
Fig. 1
The levels of interleukin (IL)-1α (A) and lipid peroxide (LPO) (B) in the content of comedones displayed statistically significant differences between non-smokers and smokers. TBA: thiobarbituric acid. *Statistically significant, p<0.05.

Fig. 2
The levels of interleukin (IL)-1α (A) and lipid peroxide (LPO) (B) in the content of comedones displayed no statistically significant differences between former smokers and current smokers. TBA: thiobarbituric acid.

Fig. 3
Comparison of interleukin (IL)-1α (A) and lipid peroxide (LPO) (B) levels concerning the content of comedones. No significant differences were evident among the six Korean acne grading system (KAGS) groups. *Classification according to the standard of KAGS. TBA: thiobarbituric acid.
References
1. Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997; 136:66–70.

2. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006; 7:281–290.
3. Rivera R, Guerra A. Management of acne in women over 25 years of age. Actas Dermosifiliogr. 2009; 100:33–37.

4. Shin MK, Kim NI. Clinical manifestation and elevated serum insulin-like growth factor-1 (IGF-1) levels with post-adolescent acne. Korean J Dermatol. 2008; 46:619–626.
6. Møller P, Wallin H, Knudsen LE. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem Biol Interact. 1996; 102:17–36.

7. Wang L, Muxin G, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evid Based Complement Alternat Med. 2007; 4:195–202.

8. Abulnaja KO. Oxidant/antioxidant status in obese adolescent females with acne vulgaris. Indian J Dermatol. 2009; 54:36–40.

9. Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008; 39:2439–2443.

10. Sung KJ, Rho YS, Choi EH, Oh JJ, Lee JH, Kim S, et al. Korean acne grading system. Korean J Dermatol. 2004; 42:1241–1247.
11. Capitanio B, Sinagra JL, Ottaviani M, Bordignon V, Amantea A, Picardo M. Acne and smoking. Dermatoendocrinol. 2009; 1:129–135.

12. Arican O, Kurutas EB, Sasmaz S. Oxidative stress in patients with acne vulgaris. Mediators Inflamm. 2005; 2005:380–384.

13. Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003; 17:663–669.

14. El-Akawi Z, Abdel-Latif N, Abdul-Razzak K. Does the plasma level of vitamins A and E affect acne condition? Clin Exp Dermatol. 2006; 31:430–434.

15. Kim WH, Park EJ, Park MW, Cho HS, Kim HJ, Kim CW, et al. Decreased hydrogen peroxide generation by neutrophils from acne patients treated with isotretinoin. Ann Dermatol. 2006; 18:59–63.

16. Klock J, Ikeno H, Ohmori K, Nishikawa T, Vollhardt J, Schehlmann V. Sodium ascorbyl phosphate shows in vitro and in vivo efficacy in the prevention and treatment of acne vulgaris. Int J Cosmet Sci. 2005; 27:171–176.

17. Wollina U, Knöll B, Prüfer K, Barth A, Müller D, Huschenbeck J. Synthetic wound dressings--evaluation of interactions with epithelial and dermal cells in vitro. Skin Pharmacol. 1996; 9:35–42.

18. Tochio T, Tanaka H, Nakata S, Ikeno H. Accumulation of lipid peroxide in the content of comedones may be involved in the progression of comedogenesis and inflammatory changes in comedones. J Cosmet Dermatol. 2009; 8:152–158.

19. Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in acne vulgaris. J Invest Dermatol. 2006; 126:2430–2437.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download