Abstract
Background
Previous clinical trials with evening primrose oil in atopic dermatitis (AD) treatment have shown different results. In addition, the optimal dose and duration of treatment with evening primrose oil have not yet been determined.
Objective
The aim of this study is to investigate the dose-response treatment effects of evening primrose oil on clinical symptoms of AD and serum concentrations of polyunsaturated fatty acids.
Methods
Forty AD patients were enrolled for the study and randomly divided into 2 groups: those who received evening primrose oil 160 mg daily for 8 weeks and those who received 320 mg of evening primrose oil twice daily for 8 weeks. We evaluated the Eczema Area Severity Index (EASI) scores of all AD patients at weeks 0, 2, 4 and 8. In addition, we measured the levels of serum fatty acids, including C16 : 0 (palmitic), C18 : 2n (linoleic), C18 : 3n (linolenic) and C20 : 4 (arachidonic acid) using gas chromatography.
Results
The serum fatty acid levels C18 : 3n and C20 : 4 were higher in the 320 mg group than in the 160 mg group, with statistical significance. After evening primrose oil treatment, EASI scores were reduced in the 2 groups. The improvement in EASI scores was greater in the 320 mg group than in the 160 mg group. There were no side effects seen in either group during the study in the 2 groups.
Evening primrose oil (EPO), which contains a group of n-6 series essential fatty acids, is approved in many countries as an adjuvant treatment for atopic dermatitis (AD). Its effective component is believed to be gamma-linolenic acid (GLA). Some authors have proposed that delta-6-desaturase is defective in AD patients1. This defect has been found to cause lower serum concentrations of GLA, dihomo-gamma-linolenic acid (DGLA), arachidonic acid (AA) and prostaglandin E1/E2 (PGE1, PGE2) in AD patients, compared to normal controls2. It has also been claimed that low concentrations of PGE1 and PGE2 play a major role in the pathogenesis of atopic disease3.
Essential fatty acids are necessary for normal epithelial permeability and are important constituents of all cellular membranes4. They are essential for survival in humans and cannot be synthesized in the human body. Common plant sources of commercially available GLA include borage (Borago officinalis), evening primrose (Oenothera biennis) and blackcurrant (Ribes nigrum)5.
Although some previous clinical trials have shown that EPO intake is effective in improving AD and diabetic neuropathy, other studies have not supported this conclusion; as such its use in AD remains controversial. Furthermore, the optimal EPO dose and treatment duration for AD patients have not yet been established. Likewise, trials to establish the dose-dependent effects have shown varying results6. There have been few studies on EPO's effects on AD, especially its dose-dependent effects on clinical symptoms and serum fatty acid levels.
The aim of this study was thus to investigate the dose-dependent effects of EPO in AD patients, in terms of both disease severity and serum fatty acid concentrations.
This study included 40 children and adolescents (24 males and 16 females) who visited the Department of Dermatology at Hallym University between June 2008 and May 2009. Their ages ranged between 2 and 15 years (mean±standard deviation [SD], 5.6±5.5 years) and they met the Hanifin and Rajka criteria7. Patients ranged in weight between 7.5 and 61 kg (mean±SD, 18.3±2.4 kg). The mean duration of eczema was 8.6±4.8 months. The mean Eczema Area Severity Index (EASI) score8 was 6.1±1.6 (range, 3.0 to 9.0). We excluded patients who had any congenital disorders, asthma or any other chronic disorders. Consent was obtained from the guardians of each patient. This study was approved by the Institutional Review Board of Kangnam Sacred Heart Hospital.
Patients were randomly divided into two treatment groups: those receiving two capsules of Evoprim (Dalim, Seoul, Korea) (Table 1), containing 40 mg of EPO per capsule, twice daily for eight weeks (160 mg group, n=20), and those who received four of the same capsules twice daily (320 mg group, n=20). There were no significant differences between the two groups in their demographic and clinical characteristics (Table 2). Patients were instructed not to change their diet during the study period. Younger patients who were unable to swallow capsules were advised to cut the capsules open. To check for compliance, each patient's remaining capsules were counted at each visit.
During the study period, patients were prohibited from receiving ultraviolet-ray treatment, nonsteroidal immunosuppressive agents (e.g., cyclosporine, azathioprine and mycophenolate mofetil), topical immunomodulators (e.g., tacrolimus and pimecrolimus), topical corticosteroids, systemic corticosteroids or any other investigational drugs. The washout phase for these treatments ranged from a minimum of three days (for topical corticosteroids and immunomodulators) to a maximum of six weeks (for ultraviolet treatment). The washout period was two weeks for systemic nonsteroidal immunosuppressants and five days for systemic corticosteroids and antihistamines. Patients were permitted to use bath oils and non-medicated emollients during the study period.
In all patients, medication compliance and adverse events were evaluated, and their EASI scores were checked at weeks 0, 2, 4 and 8. Fasting blood was taken at weeks 0 and 8. The serum levels of C16 : 0 (palmitic acid), C18 : 2n (linoleic acid), C18 : 3n (linolenic acid) and C20:4 (AA) were measured by gas chromatography (Agilent Technologies, Santa Clara, CA, USA)9. Peaks were identified by comparison with known standards.
All statistical analyses were performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as the mean±SD. Student t-test was used for comparison of the demographic and clinical characteristics between the two groups. Changes in EASI scores and fatty acid concentrations were also tested using Student's t-test. Significant correlations were analyzed by the Spearman rank correlation test. A p-value of <0.05 was considered statistically significant. Estimates of treatment differences were given with 95% confidence intervals.
The serum fatty acid levels of C16:0 (palmitic acid), C18 : 2n (linoleic acid), C18 : 3n (linolenic acid) and C20 : 4 (AA) were measured by gas chromatography (Agilent Technologies) at weeks 0 (first visit) and 8 (last visit).
In the 160 mg group, the serum levels of C16:0 (palmitic acid) changed from 0.242±0.026 nmol/L to 0.251±0.125 nmol/L. The C18 : 2n (linoleic acid) level changed from 0.282±0.035 nmol/L to 0.301±0.153 nmol/L; C18 : 3n (linolenic acid) changed from 0.005±0.002 nmol/L to 0.008±0.004 nmol/L, and those of C20:4 (AA) rose from 0.059±0.011 nmol/L to 0.079±0.025 nmol/L. All of the fatty acid levels increased, but only the levels of C18 : 3n (linolenic acid) and C20:4 (AA) showed a significant change (p=0.006 and p=0.006, respectively) (Fig. 1A).
In the 320-mg group, the serum levels of C16:0 (palmitic acid) changed from 0.252±0.037 nmol/L to 0.255±0.049 nmol/L; those of C18 : 2n (linoleic acid) changed from 0.272±0.055 nmol/L to 0.305±0.129 nmol/L, those of C18 : 3n (linolenic acid) went from 0.006±0.007 nmol/L to 0.011±0.014 nmol/L, and those of C20 : 4 (AA) rose from 0.064±0.019 nmol/L to 0.090±0.023 nmol/L. All of the fatty acid levels increased, but significant differences were, as in the previous group, only noted in the serum levels of C18:3n (linolenic acid) and C20 : 4 (AA) (p=0.001 and p=0.000, respectively) (Fig. 1B). In the 160 mg group, the serum C16 : 0 (palmitic acid) levels were 1.03 times higher at final visit than they had been at first visit; the serum C18 : 2n (linoleic acid) levels were 1.06 times higher; the serum C18 : 3n (linolenic acid) levels were 1.59 times higher, and the serum C20 : 4 (AA) levels were 1.34 times higher. In the 320 mg group, the serum C16 : 0 (palmitic acid) levels were 1.01 times higher at final visit than they had been at first visit; the serum C18 : 2n (linoleic acid) levels were 1.12 times higher; the serum C18 : 3n (linolenic acid) levels were 1.76 times higher; and the serum C20 : 4 (AA) levels were 1.4 times higher. Changes in the serum levels of C18 : 3n (linolenic acid) and C20:4 (AA) were greater in the 320 mg group than in the 160 mg group.
After GLA supplementation, AD patients showed clinical improvement (Fig. 2). In the 160 mg group, the mean EASI scores were 5.850±1.548, 5.350±1.367, 4.850±1.451 and 4.525±3.581 at weeks 0, 2, 4 and 8, respectively (Fig. 3). There were no significant differences in the EASI scores between the first and last measurements (p=0.55). In the 320 mg group, the mean EASI scores were 6.250±1.713, 5.450±1.512, 4.200±1.174 and 3.475±1.175 at weeks 0, 2, 4 and 8, respectively (Fig. 3). The differences in EASI scores between the first and final visit were statistically significant (p=0.000). The changes in EASI scores were greater in the 320 mg group than in the 160 mg group: in the 160 mg group, the mean EASI scores were 1.29 times lower at the last visit than at the first visit, whereas in the 320 mg group, they were 1.8 times lower.
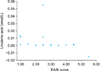
The relationships between increased serum fatty acid levels and decreased EASI scores were examined by the Spearman rank correlation test. Increased serum fatty acid levels were positively correlated with decreased EASI scores. The serum fatty acid C18:3n (linolenic acid) levels and EASI scores showed a significantly negative correlation in the 320 mg group (r=0.544, p=0.013) (Fig. 4).
EPO is a natural source of relatively high concentrations of linoleic acid and GLA. The evening primrose (O. biennis) is a plant with yellow flowers that bloom in the evening and is related to the rosebay willow herb family. Native Americans have used its mucilaginous stem and leaf juices as topical remedies to soothe cutaneous inflammations and a poultice of the plant has been used to treat bruises and minor wounds. Other sources of GLA include plant oils, such as borage seed oil, black currant seed oil, hempseed oil and spirulina (a Cyanobacterium)10.
AD may be a minor inherited abnormality of the essential fatty acid metabolism. Research in the mid-twentieth century (1930~1960) established that a deficiency of omega-6-essential fatty acids leads to an inflammatory skin condition in both animals and humans. Linoleic acid concentrations tend to be elevated in the blood, milk and adipose tissue of AD patients, whereas the concentrations of linoleic acid metabolites are substantially reduced. This suggests a reduced conversion of linoleic acid to GLA2.
Linoleic acid is converted to GLA (18 : 3, n-6) by the action of the enzyme delta-6-desaturase, and GLA is elongated into the DGLA form (20 : 3, n-3), the precursor of prostaglandin H1 (PGH1), which in turn forms PGE1 and thromboxane A1 (TXA1). DGLA is also converted to AA (20 : 4, n-6) by the action of the enzyme delta-5-desaturase. AA forms the precursor of PGE2, thromboxane A2 (TXA2) and leukotriene B4 (LTB4)11. Delta-6-desaturase production, which catalyzes linoleic acid to produce GLA, DGLA and AA, has been reported to be defective in AD patients1.
In this study, the clinical symptoms of AD patients improved after EPO treatment, and their EASI scores decreased after eight weeks of EPO administration in both the 160 mg and 320 mg groups. Many studies have found beneficial effects of EPO on AD in the pediatric age group12-14. Fiocchi et al.15 have demonstrated that fourweek GLA administration is a safe and effective adjuvant therapy for infants and children with AD. Kerscher and Korting16 have also documented that pediatric AD patients treated with GLA showed lower levels of inflammation, dryness, scaling and overall severity compared to controls. Some other studies have also confirmed the beneficial role of EPO in adult AD17,18. Andreassi et al.17 reported that adult patients receiving GLA show gradual improvement in pruritus, erythema, vesiculation and oozing. Likewise, Yoon et al.18 found that the extent of skin lesions and pruritus was markedly reduced in 14 adult patients after treatment with EPO.
Our study showed that the concentrations of C18 : 3n (linolenic) and C20 : 4 (AA) significantly increased after administration of EPO across both study groups. Previous studies have shown the effects of EPO administration on the plasma lipid profiles of AD patients1,19,20. Daily administration of 2, 4 and 6 g of EPO (Efamol; Efamol Ltd., Guildford, UK) produced a significant dose-related rise in plasma phospholipid DGLA in adult AD patients but a less consistent rise in AA1. In Japanese AD children, administration of GLA corrected the previously abnormal plasma phospholipid essential fatty acid profile20. EPO also caused dose-related increases in DGLA and AA in blood neutrophils as well as in epidermal phosphatidylcholine and phosphatidylethanolamine19. Taken together, these works show that administration of GLA exhibits significant and demonstrable effects on fatty acid profiles in AD patients.
GLA plays an anti-inflammatory and immunomodulatory role. The human body forms DGLA from GLA. DGLA is the precursor of PGH1, which in turn forms PGE 1 and TXA14. Of these, PGE1 plays a role in the regulation of immune functions and TXA1 modulates the proinflammatory properties of TXA2. Unlike other eicosanoids, DGLA cannot yield leukotrienes; however, it can inhibit the formation of proinflammatory leukotrienes from AA21. Interestingly, GLA also has an antibacterial effect. In particular, it shows bactericidal activity against Staphylococcus aureus colonization on the skin, which is a common problem in AD patients22,23.
AA derivatives, such as PGE2, TXA2 and LTB4, have proinflammatory activity. However, AA-derived prostaglandin D2 and possibly prostaglandin F2a have anti-inflammatory activity. The AA derivative of 15-hydroxyeicosatetraenoic acid also has anti-inflammatory activity22,24. Likewise, AA also has important biological functions related to the skin and is an essential constituent of membranes. The balance between DGLA and its products or between AA and its products may be a key factor in determining whether GLA supplementation produces pro-inflammatory or anti-inflammatory actions22,24. Besides being converted to anti-inflammatory products, DGLA seems to play a role in maintaining the AA in membranes in which it has a specific activity. Furthermore, the presence of high DGLA levels prevents the conversion of AA to potentially harmful metabolites22,25.
In our study, serum DGLA level data would have helped to evaluate the effects of GLA administration. However, DGLA was difficult to measure due to the absence of the standardized material. That being said, judging from the increases in GLA and AA levels, it is conceivable that DGLA, as an intermediate metabolite of omega-6-essential fatty acid metabolism, may also have increased.
No side effects were observed in our study. EPO is generally well tolerated, with only minor reported adverse effects, including gastrointestinal upset (e.g., abdominal pain, indigestion, nausea and softening of stools) and headaches26,27.
The optimal dose and administration duration of EPO have not yet been determined. In addition, the recommended dose of EPO varies among products. According to the prescribing information for the EPO (Evoprim) used in this study, the optimum oral dosage of GLA should range from 160 to 320 mg per day in pediatric AD patients and from 320 to 480 mg per day in adult AD patients. In previous clinical trials, the oral dosage of EPO in adult AD patients ranged from 160 to 640 mg per day in divided doses, with treatment durations ranging from 3 to 16 weeks28. The oral dosage of EPO in pediatric AD patients in such studies ranged from 80 to 320 mg per day in divided doses28. The results of our study showed that EPO had dose-related positive effects on clinical symptoms and serum fatty acid levels. Changes in the serum levels of C18 : 3n (linolenic acid) and C20 : 4 (AA) as well as in EASI scores were greater in the 320 mg group than in the 160 mg group. The results of this study may as such help to establish an appropriate dosing regimen for EPO and support the development of effective therapeutic methods for the treatment of AD.
After treatment with EPO, serum fatty acid levels increased, especially those of C18 : 3n (linolenic acid) and C20 : 4 (AA), whereas EASI scores decreased. In addition, changes in serum C18 : 3n (linolenic acid) and C20 : 4 (AA) levels as well as in EASI scores were greater in the 320 mg group than in the 160 mg group. It is concluded that EPO may be useful for treating AD patients in a dose-dependent manner.
Figures and Tables
 | Fig. 1Changes in serum fatty acid levels after treatment with evening primrose oil. (A) In the 160 mg group, all serum fatty acid levels increased, but only the increases in the levels of C18 : 3n (linolenic acid) and C20 : 4 (arachidonic acid) were statistically significant. *p=0.006, **p=0.006. (B) In the 320 mg group, all serum fatty acid levels increased, but significant differences were only noted in the serum levels of C18:3n (linolenic acid) and C20:4 (arachidonic acid). *p=0.001, **p=0.000. |
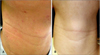 | Fig. 2A 10-year-old boy with atopic dermatitis. (A) Clinical photograph taken at first visit showing scaly erythematous patches, small erythematous papules and excoriations on the neck. (B) Clinical photograph taken eight weeks after supplementation of gamma-linolenic acid 320 mg/d shows considerably improved skin lesions. |
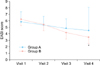 | Fig. 3Changes in Eczema Area Severity Index (EASI) scores after treatment with evening primrose oil. The mean EASI scores were 5.850±1.548, 5.350±1.367, 4.850±1.451 and 4.525±3.581 at weeks 0, 2, 4 and 8, respectively, in the 160 mg group. The mean EASI scores were 6.250±1.713, 5.450±1.512, 4.200±1.174 and 3.475±1.175 at weeks 0, 2, 4 and 8, respectively, in the 320 mg group. The difference between the first and last EASI scores was statistically significant in the 320 mg group (p=0.000). Visit 1: 0 week, Visit 2: 2 weeks, Visit 3: 4 weeks: Visit 4: 8 weeks. *p=0.000. |
ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2011-0013003 and No. 2011-0004436) and by a grant from Amore Pacific Co. Ltd., 2011.
References
1. Manku MS, Horrobin DF, Morse NL, Wright S, Burton JL. Essential fatty acids in the plasma phospholipids of patients with atopic eczema. Br J Dermatol. 1984; 110:643–648.

2. Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr. 2000; 71:1 Suppl. 367S–372S.

3. Hederos CA, Berg A. Epogam evening primrose oil treatment in atopic dermatitis and asthma. Arch Dis Child. 1996; 75:494–497.

4. Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006; 1:420–439.

5. Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, antiatherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008; 7:37.

6. Bayles B, Usatine R. Evening primrose oil. Am Fam Physician. 2009; 80:1405–1408.
7. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980; Suppl. 92. 44–47.
8. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. EASI Evaluator Group. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001; 10:11–18.

9. Raederstorff D, Meier CA, Moser U, Walter P. Hypothyroidism and thyroxin substitution affect the n-3 fatty acid composition of rat liver mitochondria. Lipids. 1991; 26:781–787.

10. Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2008; 74:447–452.

11. McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin Dermatol. 2010; 28:440–451.

12. Van Gool CJ, Zeegers MP, Thijs C. Oral essential fatty acid supplementation in atopic dermatitis-a meta-analysis of placebo-controlled trials. Br J Dermatol. 2004; 150:728–740.

13. Kawamura A, Ooyama K, Kojima K, Kachi H, Abe T, Amano K, et al. Dietary supplementation of gamma-linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J Oleo Sci. 2011; 60:597–607.

14. Van Gool CJ, Thijs C, Henquet CJ, van Houwelingen AC, Dagnelie PC, Schrander J, et al. Gamma-linolenic acid supplementation for prophylaxis of atopic dermatitis-a randomized controlled trial in infants at high familial risk. Am J Clin Nutr. 2003; 77:943–951.

15. Fiocchi A, Sala M, Signoroni P, Banderali G, Agostoni C, Riva E. The efficacy and safety of gamma-linolenic acid in the treatment of infantile atopic dermatitis. J Int Med Res. 1994; 22:24–32.

16. Kerscher MJ, Korting HC. Treatment of atopic eczema with evening primrose oil: rationale and clinical results. Clin Investig. 1992; 70:167–171.

17. Andreassi M, Forleo P, Di Lorio A, Masci S, Abate G, Amerio P. Efficacy of gamma-linolenic acid in the treatment of patients with atopic dermatitis. J Int Med Res. 1997; 25:266–274.

18. Yoon S, Lee J, Lee S. The therapeutic effect of evening primrose oil in atopic dermatitis patients with dry scaly skin lesions is associated with the normalization of serum gamma-interferon levels. Skin Pharmacol Appl Skin Physiol. 2002; 15:20–25.

19. Schäfer L, Kragballe K. Supplementation with evening primrose oil in atopic dermatitis: effect on fatty acids in neutrophils and epidermis. Lipids. 1991; 26:557–560.

20. Shimasaki H. PUFA content and effect of dietary intake of γ-linolenic acid-rich oil on profiles of n-6, n-3 metabolites in plasma of children with atopic eczema. J Clin Biochem Nutr. 1995; 19:183–192.

21. Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr. 2000; 71:1 Suppl. 352S–356S.

22. Foster RH, Hardy G, Alany RG. Borage oil in the treatment of atopic dermatitis. Nutrition. 2010; 26:708–718.

23. Lacey RW, Lord VL. Sensitivity of staphylococci to fatty acids: novel inactivation of linolenic acid by serum. J Med Microbiol. 1981; 14:41–49.

24. Ziboh VA, Miller CC, Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites. Am J Clin Nutr. 2000; 71:1 Suppl. 361S–366S.

25. Horrobin DF. Nutritional and medical importance of gamma-linolenic acid. Prog Lipid Res. 1992; 31:163–194.

26. Bamford JT, Gibson RW, Renier CM. Atopic eczema unresponsive to evening primrose oil (linoleic and gammalinolenic acids). J Am Acad Dermatol. 1985; 13:959–965.

27. Bordoni A, Biagi PL, Masi M, Ricci G, Fanelli C, Patrizi A, et al. Evening primrose oil (Efamol) in the treatment of children with atopic eczema. Drugs Exp Clin Res. 1988; 14:291–297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download