Abstract
Background
Although there have been many studies about onychomycosis due to nondermatophytic molds (NDM), few studies about etiologic agents including NDM in onychomycosis have been reported in Korea. Objective: This study investigated onychomycosis due to NDM in the Gyeongju area of Korea.
Methods
In the 10-year period from 1999~2009, we reviewed 59 patients with onychomycosis due to NDM. The etiologic agents were identified by cultures on Sabouraud's Dextrose agar with and without cycloheximide. In some cases, internal transcribed spacer sequence analysis was done. NDM isolated considered pathogens when the presence of fungal elements was identified by direct microscopy observation and in follow-up cultures yielding the same fungi.
Results
Onychomycosis due to NDM comprised 2.3% of all onychomycosis. Of the 59 patients with onychomycosis due to NDM, 84.7% were toenail onychomycosis and 15.3% were fingernail onychomycosis. The incidence rate was highest in the fifth decade (27.1%). The ratio of male to female patients was 1:1.6. The frequency of associated diseases, in descending order, was hypertension, diabetes mellitus, and cerebral hematoma. Distal and lateral subungual onychomycosis (86.4%) was the most common clinical type of onychomycosis. Aspergillus spp. was the most frequently isolated etiologic agent of onychomycosis due to NDM (83.0%). Other causative agents were Scopulariopsis brevicaulis (10.2%), Acremonium spp. (3.4%), Fusarium solani (1.7%), and Chaetomium globosum (1.7%).
Onychomycosis denotes any nail infection due to dermatophytes, nondermatophytic molds (NDM), or yeasts. The increasing prevalence of this disease may be secondary to tight shoes, increasing numbers of immunosuppressed individuals, and increased used of communal locker rooms1.
NDM are filamentous fungi that are commonly found in nature as saprophytes and plant pathogens. Because these molds are not keratolytic, unlike dermatophytes, they only live on unkeratinized intercellular cement or must take advantage of previous keratin destruction by dermatophytes, trauma, or another nail disease. For this reason, the incidence of onychomycosis due to NDM is 1.45~17.6%, which is lower than that caused by dermatophytes2,3. NDM causing onychomycosis include Scopulariopsis brevicaulis, Aspergillus species, Fusarium species, Sytalidium species, and Onychocola canadiensis1-7.
There have been numerous case reports describing onychomycosis due to S. brevicaulis8,9, Aspergillus species10-12, and Fusarium species13,14. However, onychomycosis due to NDM has only been described in one study to date15. Therefore, this study was designed to investigate clinical features and isolated pathogens of patients with onychomycosis due to NDM in the 10-year period from 1999~2009. A brief review of the literature has been included.
This study included 59 patients who clinically showed onychomycosis and were diagnosed with onychomycosis due to NDM by 15% potassium hydroxide (KOH) test and fungus culture at the Department of Dermatology, Dongguk University Gyeongju Hospital during the 10-year period from July 1999 to June 2009. Of these patients, 50 patients had toenail onychomyocosis and nine patients had fingernail onychomycosis.
The medical records of the 59 patients were retrospectively analyzed in terms of clinical features, yearly/monthly/seasonal variations in incidence, age, sex, associated disease, sites of nail involvement, and clinical type. According to the classification of Baran et al.16, we classified onychomycosis into five clinical types: distal and lateral subungual onychomycosis (DLSO), superficial white onychomycosis (SWO), proximal subungual onychomycosis (PSO), endonyx onychomycosis (EO), and total dystrophic onychomycosis (TDO).
After the nail was sterilized with 75% alcohol, we obtained the sample by scraping the hyperkeratotic nail bed with a disposable scalpel. Nail samples were microscopically studied after clearing for 30 minutes in 15% KOH. For cultures, each specimen was inoculated in three sites on Sabouraud's Dextrose agar (SDA) with and without 0.5 mg/ml cyclohexamide and incubated at room temperature for 2~4 weeks. Causative pathogens were identified based on gross and microscopic findings of colonies. In cases where this identification was not obvious, the pathogens were identified by analyzing the base sequence of the internal transcribed spacer (ITS) region. Onychomycosis due to NDM was defined according to a modification of a previously described method17, when the 15% KOH test was positive, three or more identical colonies were found in fungus cultures, and the identical fungi were identified in repeated fungus cultures.
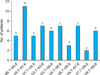
The total number of patients with onychomycosis during the study period was 2,584. Of these patients, 59 showed onychomycosis due to NDM accounting for 2.3% of all onychomycosis cases. The incidence onychomycosis due to NDM was highest during the period between July 2000 and June 2001 (n=11, 18.6%) and lowest during the period between July 2007 and June 2008 (n=2, 3.4%) (Fig. 1).
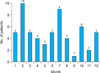
In decreasing order of frequency, 10 patients developed onychomycosis due to NDM in February; nine patients in July; six patients in October; five patients each in January, March, June, and December; four patients each in April and August; three patients in May; two patients in November; and one patient in September. Twenty patients developed onychomycosis due to NDM during the winter (December through February), 18 patients during the summer (June through August), 12 patients during the spring (March through May), and nine patients during the fall (September through November); however, the differences were not significant (Fig. 2).
Onychomycosis due to NDM occurred most commonly in patients in their 40s (27.1%), followed by those between their 50s and 60s (23.7%), and those in their 30s (13.6%). Overall, onychomycosis occurred frequently in patients between their 40s and 60s (Table 1). The male to female ratio was 1 : 1.6, with a predilection for females (23 males and 36 females) (Table 1).
Twenty (33.9%) of the 59 patients with onychomycosis due to NDM had concurrent diseases. Hypertension was most common (15.3%), followed by diabetes mellitus (n=8, 13.6%), cerebrovascular accidents (n=2, 3.4%), hyperthyroidism (n=1, 1.7%), cerebral infarction (n=1, 1.7%), breast cancer (n=1, 1.7%), chronic renal failure (n=1, 1.7%), congestive heart failure (n=1, 1.7%), and pulmonary tuberculosis (n=1, 1.7%) (Table 2).
Of the 59 patients with onychomycosis due to NDM, 50 (84.7%) had toenail onychomycosis alone and nine (15.3%) had fingernail onychomycosis alone. Of the 50 patients with toenail onychomycosis, 14 (28.0%) showed involvement of the first toenail alone and 35 (70%) showed involvement of multiple toenails including the first toenail. Of the nine patients with fingernail onychomycosis, six (66.7%) showed involvement of the first fingernail alone and two (22.2%) showed involvement of multiple fingernails including the first fingernail. The number of involved toenails was one or two in 17 patients (34.0%), five or six in 12 patients (24.0%), three or four in nine patients (18.0%), nine or 10 in eight patients (16.0%), and seven or eight in four patients (8%). The number of involved fingernails was one or two in six patients (66.7%), followed by three or four in three patients (33.3%). There were no patients with involvement of ≥5 fingernails (Table 3).
As for the clinical type of toenail onychomycosis due to NDM, DLSO was most common (n=46, 92.0%), followed by TDO (n=2, 4.0%), SWO (n=1, 2.0%) and PSO (n=1, 2.0%). As for the clinical type of fingernail onychomycosis due to NDM, DLSO was found in five patients (55.6%) and PSO in four patients (44.4%). Overall, DLSO was most common (n=51, 86.4%), followed by PSO (n=5, 8.5%), TDO (n=2, 3.4%), and SWO (n=1, 1.7%) (Table 4).
The colonies of NDM were observed on two SDA plates without cycloheximide slants at 25℃ for 2 weeks. But, there was no growth of NDM and dermatophytes on SDA with cycloheximide slants.
Of the causative organisms with toenail onychomycosis due to NDM, Aspergillus species were most commonly isolated (40/50, 80.0%), followed by S. brevicaulis (6/50, 12.0%), Acremonium species (2/50, 4.0%), F. solani (1/50, 2.0%), and C. globosum (1/50, 2.0%). Of the Aspergillus species, A. niger was the most common organism (16/50, 32.0%), followed by A. fumigatus (13/50, 26.05), A. flavus (8/50, 16.0%), A. terreus (2/50, 4.0%), and A. sydowii (1/50, 2.0%). In our patients with fingernail onychomycosis due to NDM, only Aspergillus species was isolated; A. niger was most commonly isolated (5/9, 55.6%), followed by A. fumigatus (2/9, 22.2%), A. flavus (1/9, 11.1%), and A. terreus (1/9, 11.1%). Overall, Aspergillus species was most commonly isolated (49/59, 83.0%), followed by S. brevicaulis (96/59, 10.2%), Acremonium species (2/59, 3.4%), F. solani (1/59, 1.7%), and C. globosum (1/59, 1.7%). A. niger was most commonly isolated (21/59, 35.6%), followed by A. fumigatus (15/59, 25.4%), A. flavus (9/59, 15.3%), A. terreus (3/59, 5.1%), and A. sydowii (1/59, 1.7%) (Table 5). Of the 49 Aspergillus-related cases, DLSO was most common (n=42), followed by PSO (n=4), TDO (n=2), and SWO (n=1). Of the 21 A. niger strains alone, DLSO was most common (n=15), followed by PSO (n=4), SWO (n=1), and TDO (n=1). Of the three A. terreus strains alone, DLSO was found in two cases and TDO was found in one case. One A. sydowii strain showed DLSO. Of the six S. brevicaulis strains alone, DLSO was found in five cases and PSO was found in one case. Both of the two strains of the Acremonium species showed DLSO. One F. solani strain and one C. globosum showed DLSO (Table 6).
Medically confirmed onychomycosis should be treated. This recommendation is based on several disease-specific considerations: cosmetic and functional disability, lack of spontaneous remission, impairment of health and wellbeing in elderly patients, and the need to reduce contamination in communal bathing places18. Treatment of onychomycosis due to NDM requires more time than treatment of those with onychomycosis due to dermatophytes, and some patients with onychomycosis due to NDM frequently do not respond to antifungal agents2,6,9-23. This study was performed because the incidence of onychomycosis due to NDM has recently increased3,6,24.
In this study, onychomycosis due to NDM accounted for 2.3% of all onychomycosis cases, which falls within the reported prevalence between 1.45% and 17.6%. Most of the reported incidences are higher than the present figure1-6,25. However, the incidence reported by Bonifaz et al.7 (1.49%) was lower than the present figure. The incidence was highest between July 2000 and June 2001, but there was no increasing tendency. There was no significant difference in monthly and seasonal incidence. Onychomycosis due to NDM most frequently occurred in subjects in their 40s (26.2%) and was prevalent in subjects between their 30s and 60s (84.7%), which are similar to the previous2,7. The male to female ratio was 1:1.6 with a predilection for females, consistent with previous results2,5.
In this study, concurrent diseases were found in 33.9% of patients. Hypertension was the most common concurrent disease (15.3%), followed by diabetes mellitus. Systemic diseases, such as diabetes mellitus, frequently cause onychomycosis26,27. However, onychomycosis due to NDM is not necessarily associated with systemic diseases2. In this study, toenail onychomycosis accounted for 84.7% of all cases of onychomycosis, which is lower than the results reported by Bonifaz et al.7 (96.2%) and Tosti et al.2 (89.7%), but higher than those reported by Hilmioğlu-Polat et al.5 (67.0%) and Gianni et al.6 (67.0%).
In our study, DLSO was found in 86.4% of patients, which was consistent with the results reported by Bonifaz et al.7. However, Tosti et al.2 and Moreno and Arenas3 reported that PSO with periungual inflammation was 64.4% and 76.0%, respectively. PSO was found in 44.4% of our patients with fingernail onychomycosis. Further studies on clinical types in fingernail onychomycosis are required.
In our patients with onychomycosis due to NDM, Aspergillus species were most frequently isolated (83.0%), which is consistent with the results reported by Bassiri-Jahromi and Khaksar,4 Hilmioğlu-Polat et al.5, Gupta et al.28 and Ramani et al.29. The second most commonly isolated fungi were S. brevicaulis (10.2%), Acremonium species (3.4%), F. solani (1.7%), and C. globosum (1.7%). However, several studies reported that S. brevicaulis was more frequently isolated than Aspergillus species6,7,25. Presently, of Aspergillus species, A. niger was most frequently isolated (25.6%), which coincides well with the results of previous studies5-7, followed by A. fumigatus (25.4%), A. flavus (15.3%), A. terreus (5.1%), and A. sydowii (1.7%). F. solani was also isolated in our patients. Tosti et al.2 have shown that the incidence of onychomycosis due to Fusarium species, such as F. solani and F. oxysporum, are increasing. C. globosum was isolated by analyzing the base sequence of the ITS region, as has been done previously30. In Aspergillus species, especially A. niger and S. brevicaulis, PSO was more frequently found. Similarly, Tosti et al.2 reported that PSO is more common in the Aspergillus species, S. brevicaulis, and Fusarium species.
In summary, patients with onychomycosis due to NDM accounted for a larger proportion of those with onychomycosis. In addition, various causative organisms of onychomycosis due to NDM were isolated in our patients. The results of this study suggest that further laboratory tests should be performed on patients with onychomycosis in order to identify NDM.
Figures and Tables
References
1. Verma S, Heffernan MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Superficial fungal infections: dermatophytosis, onychomycosis, tinea nigra, piedra. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1807–1821.
2. Tosti A, Piraccini BM, Lorenzi S. Onychomycosis caused by nondermatophytic molds: clinical features and response to treatment of 59 cases. J Am Acad Dermatol. 2000. 42:217–224.

4. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in tehran. Indian J Dermatol. 2010. 55:140–143.

5. Hilmioğlu-Polat S, Metin DY, Inci R, Dereli T, Kilinç I, Tümbay E. Non-dermatophytic molds as agents of onychomycosis in Izmir, Turkey - a prospective study. Mycopathologia. 2005. 160:125–128.

6. Gianni C, Cerri A, Crosti C. Non-dermatophytic onychomycosis. An understimated entity? A study of 51 cases. Mycoses. 2000. 43:29–33.

7. Bonifaz A, Cruz-Aguilar P, Ponce RM. Onychomycosis by molds. Report of 78 cases. Eur J Dermatol. 2007. 17:70–72.
8. Kim YJ, Lim SW, Suh MK, Choi JH, Bang JS, Lee JW, et al. Four cases of toenail onychomycosis caused by Scopulariopsis brevicaulis. Korean J Med Mycol. 2001. 6:97–103.
9. Kim SC, Chon TH, Kim HU. Onychomycosis due to Scopulariopsis brevicaulis. Korean J Dermatol. 2000. 38:1566–1568.
10. Lee BJ, Kim IJ, Suh SB. Two cases of onychomycosis due to Aspergillus repens. Korean J Dermatol. 1981. 19:881–886.
11. Suh SB, Byun DK, Lee KY. A case of onychomycosis by Aspergillus sydowi. Korean J Dermatol. 1968. 6:39–43.
12. Suh JC, Yeum JS, Na GY, Seo SK, Suh MK. A simple detection method to the resistance to the treatment of onychomycosis: a case report of Aspergillus sydowii onychomycosis. Ann Dermatol. 2001. 13:62–65.

13. Lim SW, Kwon SW, Suh MK, Lee HC, Choi JH, Lee JW, et al. A case of onychomycosis caused by Fusarium solani. Korean J Med Mycol. 2003. 8:21–25.
14. Kim JE, Park HJ, Lee JY, Cho BK, Kim SO. A case of onychomycosis with acute paronychia caused by Fusarium oxysporum. Korean J Med Mycol. 2002. 7:170–174.
15. Lim SW, Suh MK, Ha GY. Clinical features and identification of etiologic agents in onychomycosis. (1999-2002). Korean J Dermatol. 2004. 42:53–60.
16. Baran R, Hay RJ, Tosti A, Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998. 139:567–571.

18. Roberts DT. Onychomycosis: current treatment and future challenges. Br J Dermatol. 1999. 141:Suppl 56. 1–4.

19. Baudraz-Rosselet F, Ruffieux C, Lurati M, Bontems O, Monod M. Onychomycosis insensitive to systemic terbinafine and azole treatments reveals non-dermatophyte moulds as infectious agents. Dermatology. 2010. 220:164–168.

20. Ranawaka RR, de Silva N, Ragunathan RW. Onychomycosis caused by Fusarium sp in Sri Lanka: prevalence, clinical features and response to itraconazole pulse therapy in six cases. J Dermatolog Treat. 2008. 19:308–312.

21. Gianni C, Romano C. Clinical and histological aspects of toenail onychomycosis caused by Aspergillus spp.: 34 cases treated with weekly intermittent terbinafine. Dermatology. 2004. 209:104–110.

22. Tosti A, Piraccini BM, Lorenzi S, Iorizzo M. Treatment of nondermatophyte mold and Candida onychomycosis. Dermatol Clin. 2003. 21:491–497.
23. Gupta AK, Ryder JE, Summerbell RC. The diagnosis of nondermatophyte mold onychomycosis. Int J Dermatol. 2003. 42:272–273.

24. Gupta AK, Ryder JE, Baran R, Summerbell RC. Non-dermatophyte onychomycosis. Dermatol Clin. 2003. 21:257–268.

25. Kemna ME, Elewski BE. A U.S. epidemiologic survey of superficial fungal diseases. J Am Acad Dermatol. 1996. 35:539–542.

26. Dogra S, Kumar B, Bhansali A, Chakrabarty A. Epidemiology of onychomycosis in patients with diabetes mellitus in India. Int J Dermatol. 2002. 41:647–651.

27. Cathcart S, Cantrell W, Elewski B. Onychomycosis and diabetes. J Eur Acad Dermatol Venereol. 2009. 23:1119–1122.

28. Gupta M, Sharma NL, Kanga AK, Mahajan VK, Tegta GR. Onychomycosis: Clinico-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007. 73:389–392.

29. Ramani R, Srinivas CR, Ramani A, Kumari TG, Shivananda PG. Molds in onychomycosis. Int J Dermatol. 1993. 32:877–878.

30. Latha R, Sasikala R, Muruganandam N, Shiva Prakash MR. Onychomycosis due to ascomycete Chaetomium globosum: a case report. Indian J Pathol Microbiol. 2010. 53:566–567.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download