Abstract
Background
Photodynamic therapy (PDT) using topical aminolevulinic acid (ALA) has increasingly been used for the treatment of acne vulgaris and several studies have shown its clinical efficacy. However, ALA-PDT needs a relatively long incubation period and is frequently associated with adverse effects. Indole-3-acetic acid (IAA) has been introduced as a new photosensitizer for the treatment of acne in recent study. IAA-PDT requires only a short incubation period and the procedure is relatively painless in contrast to ALA-PDT.
Methods
Twenty-five patients with facial acne lesions were enrolled in this study. IAA-PDT was performed for five sessions at 1-week intervals (week 0~4). IAA was treated with 15 minute occlusion, and green light was given for 15 minutes. Clinical efficacy was determined by evaluating acne lesion counts, severity grading, and the Dermatology Life Quality Index (DLQI) at week 0, 2, 4, and 5. Sebum secretion and erythema index was measured by Sebumeter and Mexameter, respectively, at baseline and one week after each treatment session (week 1~5). Histopathological examination was performed at baseline and week 5. Adverse effects were recorded throughout the study.
Results
All the patients completed the study. Numbers of both inflammatory and non-inflammatory acne lesions were significantly decreased. Acne severity grade and the DLQI showed significant reduction. Sebum secretion and erythema were also reduced. Histopathological examination showed a reduction in inflammatory reactions. No adverse effects were observed except for transient pruritus in one patient.
Acne vulgaris is a common skin disorder causing chronic inflammation of the pilosebaceous units. Increased sebum production, abnormal keratinization of keratinocytes, colonization by Propionibacterium acnes and inflammation are thought to be the major pathogenic factors. The standard treatments for acne vulgaris include topical antimicrobials, topical retinoids, oral antibiotics, and oral isotretinoin. However, the effects of conventional therapies are limited due to antibiotic resistance and adverse effects such as irritation and teratogenicity of isotretinoin1,2. Therefore, there is a need for the development of alternative treatments for acne.
Currently, several authors have reported on the efficacy of photodynamic therapy (PDT) for acne3-5. PDT uses a photosensitizing drug activated by light, and causes cell destruction by the generation of reactive oxygen species3. 5-aminolevulinic acid (ALA) is commonly used and ALA-induced protoporphyrin IX has been shown to accumulate not only in the epidermal cells but also in the pilosebaceous units6,7. Although its specific mechanism involved has not been fully identified, it has been postulated that ALA-PDT causes phototoxic injury of sebaceous glands and inhibits sebum production, photodynamic killing of P. acnes, and reduction of follicular obstruction3-7. However, adverse effects such as pain, burning, stinging, erythema, exfoliation, and post-inflammatory hyperpigmentation are commonly observed in ALA-PDT3-5. Moreover, topical ALA has to be applied for a relatively long period under occlusion, since ALA requires time to convert into protoporphyrin IX and penetrate into the skin. In addition, strict photoprotection is required after the treatment procedure to avoid phototoxicity8.
Recently, indole-3-acetic acid (IAA) has been introduced as a new photosensitizer for PDT of acne9. IAA is a member of the group of phytohormones called auxins10. IAA alone is non-toxic in humans, however it can be activated after oxidative decarboxylation by horseradish peroxidase (HRP) and becomes cytotoxic11,12. IAA can be activated not only by HRP but also by visible and ultraviolet light9,13. Among various wavelengths of visible light, green light (520 nm) activated IAA most effectively9. The pilot study of IAA-PDT using green light has been shown to be painless and does not require long incubation periods or photoprotection after the procedure in contrast to ALA-PDT9.
The purpose of this study was to evaluate the efficacy and tolerability of PDT using IAA and green light in the treatment of acne.
This study was performed between January 2010 and May 2010. The study was approved by the Institutional Review Board at our hospital and informed consent was obtained from all patients. Patients older than 16 years with acne lesions on the face were included. Patients were excluded if they had used any acne treatment during the previous 4 weeks or had any other skin diseases on the face. Pregnant and lactating women were also excluded. Twenty-five patients with facial acne lesions were enrolled.
The patients were treated once a week for five consecutive weeks. Before application of IAA (0.015%), the skin was gently cleansed and IAA was applied for 15 minutes under occlusion. Then, green light (520 nm, 9 J/cm2) was illuminated for 15 minutes.
All patients were evaluated at baseline (week 0) and one week after each treatment session (week 1~5). During each visit, standardized frontal and bilateral 45° side photographs were taken. The numbers of inflammatory (papules, pustules, nodules and cysts) and non-inflammatory (comedones) acne lesions were counted and severity was assessed based on the Korean Acne Grading System14 at week 0, 2, 4, and 5. The severity score (grades 1~6) was as follows: grade 1, <10 papules; grade 2, 11~30 papules; grade 3, >31 papules and <10 nodules; grade 4, 11~20 nodules; grade 5, 21~30 nodules; and grade 6, >31 nodules14.
Patients were asked to complete the Dermatology Life Quality Index (DLQI) questionnaire15 at week 0, 2, 4, and 5. The DLQI is one of the most widely used instruments for assessing skin-related quality of life (QoL). It consists of 10 questions, and each item is scored on a four-point scale (0~3 points). Higher scores indicate greater impairment of the patient's QoL.
Objective skin measurements were performed at each follow-up visit. Erythema index of the acne lesions on both cheeks and forehead was measured with a Mexameter (MX-18; Courage & Khazaka Electronic GmbH, Cologne, Germany). Sebum secretion on both cheeks and forehead was also measured with a Sebumeter® (SM 815; Courage & Khazaka) in a room with constant temperature (22~23℃) and humidity (relative humidity 40~45%). The mean values of erythema index and sebum secretion were calculated by averaging the values obtained from both cheeks and forehead.
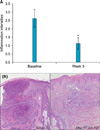
At baseline and 1 week after the final treatment session (week 5), a 2 mm punch biopsy was performed from most acne lesions in 8 patients. Specimens were sectioned and stained with hematoxylin and eosin. Severity of inflammation was graded using a 0~3 scale (grade 0: <5%, 1: 5~30%, 2: 31~70%, 3: >70%).
Adverse effects were recorded throughout the study. The investigator graded the degree of erythema, scaling, hyperpigmentation, pruritus and pain at each visit, using a 0~3 scale (0, none; 1, mild; 2, moderate; 3, severe).
Twenty-five acne patients were enrolled in the study. All patients were Korean women, and their mean age was 25.68±0.88 years (age range: 18~34 years). All the patients completed the study.
Median reduction in numbers of inflammatory and non-inflammatory acne lesions from baseline to week 2, 4, and 5 are shown in Fig. 1A, B. There was a significant reduction in inflammatory acne lesion counts from week 2. Non-inflammatory lesion counts also showed significant reductions at week 2 and 5. The representative photographs showing the improvement observed on treated patient are shown in Fig. 1C. Acne severity assessed by the Korean Acne Grading System also showed significant reduction from 1.4 at baseline and 1.16 and 1.08 at week 2 and 5 (p=0.011 and 0.008, respectively). The mean DLQI score at baseline was 7.00 and significantly decreased to 5.92, 4.64, and 4.56 at week 2, 4 and 5, respectively. No significant adverse effects were reported. Only one subject had mild pruritus, which was self-limiting.
The mean value of erythema index of the acne lesions showed a significant reduction throughout the study period from week 2 (Fig. 2). Sebum secretion showed significant reduction at week 5 (Fig. 3). Histopathological examination showed a significant reduction in inflammation at week 5 compared to baseline (Fig. 4).
IAA is a plant growth hormone and plays an important role in plant cell division, elongation and differentiation10. IAA alone is a non-toxic substance, but can produce reactive oxygen species and a variety of free radicals when oxidized by HRP11. Recent reports suggest that an IAA/HRP combination is cytotoxic to mammalian cells, and can be used as novel cancer therapy11,12,16,17. Furthermore, light activated IAA has shown its anti-cancer activity in several studies13,18,19. In addition to its potential as an anticancer drug, a recent study by Na et al.9 showed that IAA could be used as a novel photosensitizer for PDT of acne. Green light was chosen for the light source, since it activated IAA most effectively. Even low fluence (9 J/cm2) of green light was sufficient to activate IAA. The combination of IAA and green light significantly decreased the colony count of P. acnes and S. aureus. Histological examination in mice back skin revealed that the combination of IAA and green light destroyed follicular epithelial cells. Fourteen patients were treated with a combination of IAA and green light 3 times at 2-week intervals and inflammatory lesion counts and sebum excretion significantly reduced9.
In the present study, we conducted IAA-PDT using green light 5 times at 1-week intervals, which was different from a previous study9. We observed significant improvement through multidisciplinary approaches. Both inflammatory and non-inflammatory acne lesions were significantly decreased. The patients showed rapid improvement from week 2. Erythema index and sebum secretion were also significantly reduced after 5 treatment sessions. Additionally, histopathological examination was performed in 8 patients and the intensity of inflammation significantly decreased. Clinical and histological improvement shown in our study may be the effect of IAA itself or the result of antimicrobial activities on P. acnes9.
The strength of IAA-PDT is that it could be done very easily with only a short incubation period and does not require photoprotection. Furthermore, we experienced that the procedure was completely painless in contrast to ALA-PDT.
One limitation of our study is that there were no control subjects. The lack of long-term follow-up data was another limitation. Therefore, further controlled studies are required to determine an effective regimen to ensure a long-lasting result. In conclusion, the combination of IAA and green light was shown to be a very simple, safe and effective treatment option for acne.
Figures and Tables
References
1. Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, Leyden JJ, Global Alliance to Improve Outcomes in Acne, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009. 60:Suppl 5. 1–50.

3. Hongcharu W, Taylor CR, Chang Y, Aghassi D, Suthamjariya K, Anderson RR. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000. 115:183–192.

4. Itoh Y, Ninomiya Y, Tajima S, Ishibashi A. Photodynamic therapy of acne vulgaris with topical delta-aminolaevulinic acid and incoherent light in Japanese patients. Br J Dermatol. 2001. 144:575–579.

5. Pollock B, Turner D, Stringer MR, Bojar RA, Goulden V, Stables GI, et al. Topical aminolaevulinic acid-photodynamic therapy for the treatment of acne vulgaris: a study of clinical efficacy and mechanism of action. Br J Dermatol. 2004. 151:616–622.

6. Divaris DX, Kennedy JC, Pottier RH. Phototoxic damage to sebaceous glands and hair follicles of mice after systemic administration of 5-aminolevulinic acid correlates with localized protoporphyrin IX fluorescence. Am J Pathol. 1990. 136:891–897.
7. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992. 14:275–292.
8. Moseley H, Ibbotson S, Woods J, Brancaleon L, Lesar A, Goodman C, et al. Clinical and research applications of photodynamic therapy in dermatology: experience of the Scottish PDT Centre. Lasers Surg Med. 2006. 38:403–416.

9. Na JI, Kim SY, Kim JH, Youn SW, Huh CH, Park KC. Indole-3-acetic acid: a potential new photosensitizer for photodynamic therapy of acne vulgaris. Lasers Surg Med. 2011. 43:200–205.

10. Goldsmith MH. Cellular signaling: new insights into the action of the plant growth hormone auxin. Proc Natl Acad Sci U S A. 1993. 90:11442–11445.

11. Folkes LK, Wardman P. Oxidative activation of indole-3-acetic acids to cytotoxic species- a potential new role for plant auxins in cancer therapy. Biochem Pharmacol. 2001. 61:129–136.

12. Kim DS, Jeon SE, Park KC. Oxidation of indole-3-acetic acid by horseradish peroxidase induces apoptosis in G361 human melanoma cells. Cell Signal. 2004. 16:81–88.

13. Kim DS, Kim SY, Jeong YM, Jeon SE, Kim MK, Kwon SB, et al. Light-activated indole-3-acetic acid induces apoptosis in g361 human melanoma cells. Biol Pharm Bull. 2006. 29:2404–2409.

14. Sung KJ, Rho YS, Choi EH, Oh JJ, Lee JH, Kim S, et al. Korean acne grading system. Korean J Dermatol. 2004. 42:1241–1247.
15. Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008. 159:997–1035.

16. Wardman P. Indole-3-acetic acids and horseradish peroxidase: a new prodrug/enzyme combination for targeted cancer therapy. Curr Pharm Des. 2002. 8:1363–1374.
17. Greco O, Folkes LK, Wardman P, Tozer GM, Dachs GU. Development of a novel enzyme/prodrug combination for gene therapy of cancer: horseradish peroxidase/indole-3-acetic acid. Cancer Gene Ther. 2000. 7:1414–1420.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download