Abstract
Dermatomyositis (DM) is an idiopathic inflammatory process characterized by proximal muscle weakness and cutaneous lesions, such as the Gottron's sign, heliotrope rash, and erythematous photosensitive rash. Administration of systemic agents for the treatment of underlying systemic diseases leads to remission of the cutaneous lesions in many cases. However, cutaneous lesions may remain refractory to treatment. Pimecrolimus is a calcineurin inhibitor with combined anti-inflammatory and immunomodulatory activity. It has high affinity to the skin and low permeation potential, even in patients with acute skin inflammation and in those undergoing post-topical corticosteroid therapy. We herein report two DM patients whose cutaneous lesions were refractory to conventional treatment but showed dramatic response to topical pimecrolimus. The clinical outcomes suggest that topical pimecrolimus may be a good therapeutic alternative for the management of the cutaneous lesions of DM.
Dermatomyositis (DM) is a rare inflammatory myopathy associated with characteristic skin lesions and muscular weakness1. Administration of systemic agents, such as corticosteroids, hydroxychloroquine, methotrexate, mycophenolate mofetil, and/or intravenous immunoglobulins for the treatment of myopathy lead in many cases to remission of the cutaneous lesions. Nevertheless, cutaneous lesions may sometimes exhibit discordant response to therapy for myopathy and can continue to be refractory to treatment2.
Pimecrolimus is a calcineurin inhibitor with combined anti-inflammatory and immunomodulatory activity3. This is the first report topical pimecrolimus was used for the treatment mode of cutaneous lesions of DM. We describe a patient with classic DM and a patient with clinically amyopathic DM (CADM). In both cases, cutaneous lesions improved markedly after treatment with topical pimecrolimus.
A 33 year-old woman with DM was referred to our dermatologic clinic with an erythematous photosensitive rash over her face, neck, hands, shoulder and back. She showed the shawl sign (Fig. 1A), and Gottron's sign (Fig. 1B). She had been treated with methotrexate (10 mg/week p.o.), hydroxychloroquine sulfate (400 mg/day p.o.), prednisolone (15 mg/day p.o), and cyclosporin (50 mg/day p.o.) for the previous 2 months at a rheumatology clinic. Despite marked improvement in muscle weakness, the cutaneous lesions remained active. As an alternative treatment, topical application of pimecrolimus cream 1% was attempted over the affected areas twice daily. Four months later, she demonstrated good response, especially the shawl sign (Fig. 1C), while the Gottron's sign showed mild improvement (Fig. 1D). After ongoing therapy for another 6 months, all cutaneous lesions resolved (Fig. 1E, F), and she stopped applying topical pimecrolimus. During a follow-up period of 4 months, there did not experience relapse.
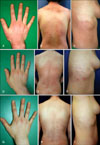
A 43 year-old woman presented with a 5-month history of periorbital rash and violaceous papules over her proximal interphalangeal and metacarpophalangeal joints (Fig. 2A), periungual erythema, and pruritic poikilodermatous erythema on her nape (Fig. 2B) and trunk (Fig. 2C). She had no history of muscle weakness. A biopsy specimen obtained from her back reavealed histopathological findings compatible with DM. She did not develop muscle weakness and had no serum muscle enzyme abnormalities for 7 months. Based on the history, laboratory and histopathological findings, a diagnosis of CADM was made. She was subsequently treated with methotrexate (15 mg/week, p.o.), hydroxychloroquine sulfate (400 mg/day p.o.), and prednisolone (15 mg/day p.o.), topical corticosteroids and sunscreens, and her cutaneous lesions temporarily subsided but subsequently showed repetitive relapse. Twice daily application of topical pimecrolimus cream 1% was initiated. Six months after starting this treatment, the Gottron's sign on her fingers showed moderate improvement (Fig. 2D). Almost all poikilodermatous erythematous lesions on her nape and trunk showed significant improvement with no associated adverse effects (Fig. 2E, F). After 1 year of treatment, the cutaneous lesions almost resolved, and topical pimecrolimus treatment was subsequently stopped. Throughout a follow-up period of 1 year after stopping treatment, the cutaneous lesions maintained their improvement (Fig. 2G, H, I).
DM is an idiopathic inflammatory process manifested by proximal muscle weakness and characteristic cutaneous lesions. The term classic DM refers to the concurrence of myositis resulting in clinically significant proximal muscle weakness and hallmark inflammatory skin lesions developed in specific anatomical distribution. On the contrary, CADM solely describes hallmark cutaneous manifestations of DM for prolonged periods (6 months or longer) without clinically evident muscle weakness4.
Cutaneous lesions may be the major manifestation of DM1. Nevertheless, cutaneouslesions of DM are sometimes refractory to several therapeutic modalities2. Dawkins et al.2 examined 35 patients, and found that 15 of them experienced resistant cutaneous lesions despite reduction in their muscle disease by oral corticosteroids and anti-malarials, followed by oral methotrexate.
Calcineurin inhibitors such as pimecrolimus and tacrolimus mainly inhibit the action of calcineurin. They act by binding to isomerase macrophilin 12 to form complexes that block serine-threonine phosphatase calcineurin, a protein that physiologically dephosphorylates, and thereby activates, the cytoplasmic subunits of the nuclear factor of the activated T cells (NF-AT). Thus, NF-AT cannot enter the nucleus to form a complex with the nuclear subunit, and therefore cannot interact with the promoter regions of many cytokine genes, including interleukin-2 (IL-2), a key regulator of T cell proliferation and differentiation, and others such as IL-3, IL-4, IL-5, interferon γ, and TNF-α3,5. Pimecrolimus is an ascomycin immunomodulating macrolactam. The pharmacologic activity of pimecrolimus is known to be more selective than that of tacrolimus, because it does not affect the differentiation, maturation or functions of Langerhans cells and does not induce apoptosis6. In addition, pimecrolimus is more lipophilic than tacrolimus, therefore it has higher affinity to the skin and lower permeation potential3,7. Pimecrolimus cream has been shown to be effective in several cutaneous inflammatory diseases, such as atopic dermatitis, inverse psoriasis, vitiligo, oral lichen planus, and recently, cutaneous lupus eyrthematosus8,9. Although topical tacrolimus has been successfully used in the treatment of cutaneous lesions of DM, as have been reported since 200210, there has been no report on topical pimecrolimus treatment of cutaneous lesions of DM.
We used topical pimecrolimus to treat cutaneous lesions of classic DM and CADM for the first time, and witnessed remarkable improvement in the poikilodermatous erythema on the trunk and the photosensitive rash on the face. The clinical outcomes suggest that topical pimecrolimus may be an efficacious alternative for the management of cutaneous lesions of DM. However, careful long-term follow-up is mandatory in these patients, and controlled clinical trials are warranted to validate our findings.
Figures and Tables
Fig. 1
(A) Patient 1 with the shawl sign before treatment. (B) Gottron's sign before treatment. (C, D) Two months later after treatment with topical pimecrolimus. (E, F) Ten months later after treatment with topical pimecrolimus.
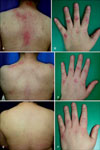
Fig. 2
(A) Patient 2 with the Gottron's sign before treatment. (B) Poikilodermatous erythematous patch on the nape and trunk before treatment. (C) Poikilodermatous erythematous patch on the right flank before treatment. (D, E, F) Three months after treatment with topical pimecrolimus. (G, H, I) One year later after stopping topical pimecrolimus treatment.
References
2. Dawkins MA, Jorizzo JL, Walker FO, Albertson D, Sinal SH, Hinds A. Dermatomyositis: a dermatology-based case series. J Am Acad Dermatol. 1998. 38:397–404.

4. Sontheimer RD, Costner MI. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Dermatomyositis. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1536–1553.
5. Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996. 80:S40–S45.

6. Hoetzenecker W, Meingassner JG, Ecker R, Stingl G, Stuetz A, Elbe-Bürger A. Corticosteroids but not pimecrolimus affect viability, maturation and immune function of murine epidermal Langerhans cells. J Invest Dermatol. 2004. 122:673–684.

7. Meingassner JG, Aschauer H, Stuetz A, Billich A. Pimecrolimus permeates less than tacrolimus through normal, inflamed, or corticosteroid-pretreated skin. Exp Dermatol. 2005. 14:752–757.

8. Rodríguez-Martín M, Sáez-Rodríguez M, Carnerero-Rodríguez A, Rodríguez-García F, Cabrera de Paz R, Sidro-Sarto M, et al. Treatment of perioral dermatitis with topical pimecrolimus. J Am Acad Dermatol. 2007. 56:529–530.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download