Abstract
Background
Through differentiation processes, keratinocytes provide a physical barrier to our bodies and control skin features such as moisturization, wrinkles and pigmentation. Keratinocyte differentiation is disturbed in several skin diseases such as psoriasis and atopic dermatitis.
Objective
The aim of this study is to evaluate the keratinocyte differentiation-enhancing effect of rose absolute oil (RAO).
Methods
Primary cultured human normal keratinocytes were treated with RAO, and differentiation then checked by the expression of marker genes.
Results
RAO did not induce cytotoxicity on cultured keratinocytes at a dose of 10µM. The level of involucrin, an early marker for keratinocyte differentiation, was significantly increased by RAO. Concomitantly, RAO increased involucrin promoter activity, indicating that RAO increased involucrin gene expression at the mRNA level. Furthermore, RAO increased the level of filaggrin in cultured keratinocytes, and in the granular layer of mouse skin. In line with these results, RAO decreased the proliferation of keratinocytes cultured in vitro. When RAO was applied topically on the tape-stripped mouse skins, it accelerated the recovery of disturbed barrier function.
In the epidermis, keratinocytes bear most responsibility for maintaining structure and homeostasis. Epidermal keratinocytes provide the rigid stratified structure through a sophisticated differentiation program1,2. Keratinocyte differentiation involves the process of cell cycle arrest and the onset of expression of numerous genes, resulting in, characteristically, 4 layers of epidermis (stratum basale, stratum spinosum, stratum granulosum and stratum corneum)3,4. The transition from basal cells to corneocytes is a complex process that requires the simultaneous activation and inactivation of a wide variety of genes5. It has been established that many genes such as involucrin, loricrin and filaggrin are expressed in a temporally regulated manner during keratinocyte differentiation6.
Dysregulated keratinocyte differentiation is also closely related with several skin diseases including psoriasis and atopic dermatitis7-9. Interestingly, such complex inflammatory skin diseases are associated with hyperproliferation of keratinocytes and disruption of skin barrier function, resulting in exacerbation of immunologic reaction and inflammation. Concordantly, disruption of skin barrier function leads to excessive dry skin, which may be another exacerbating factor for differentiation-related skin diseases10. To date, major modalities for these skin diseases are linked to the inactivation of immune reactions, such as cyclosporine A, tacrolimus and pimecrolimus11. Additionally, much evidence indicates that moisturization of skin has a beneficial effect on reducing disease status and enhancing skin texture12. It is known that the final products of keratinocyte differentiation, such as filaggrin, provide natural moisturizing properties, thereby allowing for the maintenance of healthy skin13-15. Thus, we can envisage the usage of therapeutic agents which enhance keratinocyte differentiation in conjunction with first-line treatment agents such as immunosuppressives. In this study, using an in vitro culture system, we demonstrate that rose absolute oil (RAO) can enhance keratinocyte differentiation, suggesting that RAO can be used to strengthen skin texture.
Normal human skin samples were obtained from circumcisions, in accordance with a process approved by the ethical committee of Chungnam National University Hospital. Keratinocytes were primary cultured as previously reported16. Briefly, specimens were sterilized in 70% ethanol, minced, and then treated with dispase overnight at 4℃. The epidermis was separated and placed in a solution containing 0.05% trypsin and 0.025% ethylenediaminetetraacetic acid (EDTA) at 37℃ for 15 min. After vigorous pipetting, cells were pelleted and resuspended in keratinocyte-serum free medium (K-SFM) supplemented with bovine pituitary extract and recombinant human epidermal growth factor (Invitrogen, Grand Island, NY, USA).
Keratinocytes (2×105) were seeded on 12-well culture plates and incubated overnight. After treatment with RAO, cells were replenished with fresh medium. After incubation for the indicated time points, cells received 2 mg/ml MTT solution and were incubated for a further 4 h. The medium was removed and the resulting formazan crystal was solubilized in 100µl of dimethylsulfoxide (DMSO). The optical density at 540 nm was determined using an enzyme-linked immunosorbent assay (ELISA) reader.
Cells were lysed in Proprep solution (Intron, Daejeon, Korea). Total protein was measured using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples were run on sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred onto nitrocellulose membranes and incubated with appropriate antibodies. Blots were then incubated with peroxidase-conjugated secondary antibodies, visualized by enhanced chemiluminescence (Intron, Daejeon, Korea). The following primary antibodies were used in this study: involucrin (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), filaggrin (Covance, Princeton, NJ, USA), and actin (Sigma, St. Louis, MO, USA).
For creation of involucrin-luc and loricrin-luc reporter adenoviruses, genomic DNA isolated from keratinocytes was used as a template for polymerase chain reaction (PCR). Primer sequences were as follows: involucrin promoter, 5'-CTCCATGTGTCATGGGATATG and 5'-TCAACCTGAAAGACAGAAGAG. The amplified DNA fragment was subcloned into pENT/GL3 vector that contained attL sites for site-specific recombination with a Gateway destination vector. The replication-incompetent adenoviruses were created using Virapower adenovirus expression system (Invitrogen, Grand Island, NY, USA) according to the method previously described17. Briefly, site-specific recombination between entry vector and adenoviral destination vector was achieved by LR clonase. The resulting adenoviral expression vector was then transfected into 293A cells using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA). Cells were grown until 80% cytopathic effect (CPE) was seen, then harvested for preparation of recombinant adenovirus.
Keratinocytes were grown at 50% confluency in 12-well culture plates, transduced with reporter adenovirus. After adenoviral transduction, cells were replenished with fresh medium and treated with RAO. Cells were further incubated for 48 h, and then cellular extracts were prepared using cell lysis buffer. Luciferase activities were determined using Luciferase assay system (Promega, Madison, WI, USA), according to the recommended protocol.
Paraffin sections of skin specimens were de-waxed, re-hydrated and washed 3 times with phosphate buffered saline (PBS). Sections were then incubated with proteinase K (Dako, Carpinteria, CA, USA) for 5 min at 37℃, and treated with H2O2 for 10 min at room temperature, blocked in 0.1% Tween-20, 1% bovine serum albumin in PBS for 30 min, and followed by reaction with anti-filaggrin antibody (Abcam, Cambridge, MA, USA) for 1 h. Sections were incubated sequentially with peroxidase-conjugated secondary antibody and visualized with Chemmate envision detection kit (Dako, Carpinteria, CA, USA). Sections without primary antibody were used as negative controls.
For [3H]thymidine uptake assay, keratinocytes were seeded in a 60-mm culture dish and treated with 1µCi of [3H]thymidine (Amersham, Buckinghamshire, UK). Following incubation for the indicated time point, cells were washed twice with PBS and incubated with 0.1 N NaOH at room temperature. Radioactivity in cell lysates was measured by liquid scintillation counter.
Seven-week-old female hairless mice were purchased from Orient Bio Inc. (Seongnam, Korea). Skin barrier was disturbed by tape-stripping, then RAO was topically applied. RAO was dissolved in 70% polyethylene glycol and 30% ethanol. Transepidermal water loss (TEWL) was measured using an evaporimeter (Tewameter TM210, Courage+Khazaka, Koln, Germany), as previously described18.
To investigate the cytotoxicity of RAO on keratinocytes, we treated normal human epidermal keratinocytes with RAO serially. After treatment for 3 days, cell viability was checked by MTT assay. As shown in Fig. 1, RAO showed no cytotoxicity up to a dose of 10µg/ml. Next, we determined the effect of RAO on keratinocyte differentiation by examining the protein level of involucrin, an early marker for differentiation. Calcium, the best-known differentiation inducer, was included as a positive control. After treatment for 3 days, the protein level for involucrin was markedly increased by RAO in a dose-dependent manner (Fig. 2A). To investigate whether RAO affected the involucrin expression at the promoter level, we tested the involucrin promoter activity using a recombinant adenovirus harboring reporter construct in which sequences 3.0 kb upstream of the translation start site of the involucrin gene were fused to the luciferase gene. As expected, RAO of triplicate measurements. induced luciferase activity in a dose-dependent manner, similar to that of calcium treatment (Fig. 2B). Filaggrin, the late differentiation marker for keratinocyte differentiation, was also checked by Western blotting, after long-term treatment with RAO. Interestingly, RAO treatment resulted in significant induction of filaggrin after 7 day treatment (Fig. 3). To further investigate the effect of RAO in vivo, we topically applied RAO on mouse skin. After 7 day treatment, the expression of filaggrin was evaluated by immunohistochemistry. RAO treatment led to a significant increase of filaggrin in the granular layer of mouse skin, potentiating its keratinocyte differentiation-enhancing activity.
As the differentiation process takes place along a pathway that leads to concomitant cell cycle arrest, we next evaluated the effect of RAO on cell growth using [3H]thymidine uptake assay. As anticipated, RAO treatment led to the retardation of cell growth, consistent with the results of enhancing keratinocyte differentiation (Fig. 4). TEWL is a well established indicator that reveals the disturbance of skin barrier function. To investigate the effect of RAO on skin barrier function, we disturbed mouse skin barrier with a tape-stripping method. When topically applied, significant acceleration of barrier recovery was observed after 4 h treatment of RAO. To enhance the keratinocyte differentiation further, we also formulated a RAO-containing complex in which RAO (0.1%), oleic acid (0.1%), oleanolic acid (0.05%), urocanic acid (0.01%), acetylhexapeptide (0.01%) and ceramide (0.1%) were additionally included. Interestingly, this RAO complex also markedly accelerated the barrier recovery (Fig. 5A). In line with these results, immunohistochemistry showed that filaggrin expression was significantly increased in RAO- and RAO complex-treated groups (Fig. 5B).
In epidermis, basal layer keratinocytes proliferate and move upwards, with the differentiation process beginning in the suprabasal layer and culminating in fully differentiated dead cells on the surface19. The resultant cornified layers function as a physical barrier to protect the organism from the environment. It is well known that much of this barrier function is provided by the cornified cell envelope (CE), a specialized insoluble structure formed beneath the plasma membrane in terminally differentiated stratified squamous epithelium20. The CE is built by the cross-linking of various components including filaggrin, involucrin, loricrin and small proline-rich (SPR) proteins in the plasma membrane21,22. Among them, filaggrin aggregates with keratins to form macrofilaments after cleavage of polymeric proteins into monomers. Filaggrin monomers are also degraded into small amino acid molecules, which function as natural moisturizing factors in the stratum corneum and as UV radiation filters23. Interestingly, it has been shown that wrinkle formation is closely related with thinning of epidermis together with filaggrin reduction24. It has also been suggested that stress to the skin barrier promotes the pigmentation process25. Furthermore, filaggrin expression is elevated in reepithelialized epithelium during human cutaneous wound healing26. In this regard, it could be suggested that strengthening of skin texture by enhancing keratinocyte differentiation may also influence wrinkle formation, pigmentation and skin regeneration.
Many studies indicate that disruption of the cutaneous barrier is closely associated with skin disorders such as psoriasis and atopic dermatitis. But controversy still remains as to whether the cause of differentiation-related skin diseases is primarily dependent on perturbation of skin barrier or the onset of abnormal inflammatory immune reaction27. Despite the debatable evidence, however, it is generally accepted that skin manifestations are directly linked to the dysregulated keratinocyte differentiation, including epidermal hyperproliferation, changes in keratin compositions, and down-modulation of the cornified envelope protein filaggrin28,29. Therefore, an approach that enhances keratinocyte differentiation, thereby normalizing the skin texture, is an attractive method for curing differentiation-related skin diseases. In this study, we attempted to validate the keratinocyte differentiation-enhancing effect of a candidate agent using primary cultured keratinocytes. We demonstrated that RAO is able to enhance keratinocyte differentiation and inhibit cell proliferation. In addition, RAO upregulated filaggrin production and accelerated the recovery of disturbed skin barrier, suggesting that it is able to exert beneficial effects on skin texture through the increase of natural moisturizing factors.
The main component of RAO is phenethyl alcohol (C6H5CH2CH2OH), a phenolic compound. The phenethyl alcohol can be obtained from a variety of plant sources including rose, carnation, hyacinth and orange blossom. It has been demonstrated that this phenolic compound has antioxidant and antibacterial effects30. Interestingly, several studies indicate that antioxidants have profound effects on keratinocyte differentiation. For example, N-acetyl L-cysteine (NAC) induces a 10-fold more rapid differentiation in normal primary keratinocytes, arrests the cell cycle and promotes cytoskeletal reorganization31. Other evidence indicates that the antioxidant epigallocatechin gallate (EGCG) promotes keratinocyte differentiation32. In this context, we speculate that the keratinocyte differentiation-promoting effect of RAO may be related to its antioxidant potential. However, the precise mechanism by which phenethyl alcohol effects keratinocyte differentiation remains to be elucidated.
In our study, RAO treatment resulted in increase of involucrin promoter activity. Since phenolic antioxidant triggers activation of mitogen-activated protein kinase (MAPK) signaling and induction of phase II/III drug metabolizing enzymes, it may be that RAO influences the intracellular signaling cascades related with keratinocyte differentiation. Elucidation of precise intracellular signaling induced by RAO will be an interesting topic for further study.
In conclusion, RAO has the potential to enhance keratinocyte differentiation and inhibit cell proliferation. Our results suggest that RAO may be applicable for the control of skin texture and keratinocyte differentiation-related skin diseases in conjunction with first-line treatments.
Figures and Tables
 | Fig. 1Cytotoxicity of rose absolute oil (RAO). Normal human epidermal keratinocytes were treated with RAO at the indicated concentrations for 3 days. Cell viability was measured by MTT assay. The mean values±SEM are averages of triplicate measurements. |
 | Fig. 2Effect of rose absolute oil (RAO) on involucrin expression in keratinocytes. (A) Normal human epidermal keratinocytes were treated with RAO at the indicated concentrations for 3 days. Calcium (1.2 mM) was included as a positive control for keratinocyte differentiation. Cellular proteins were prepared and protein level for involucrin was evaluated by Western blot. Actin was used as a loading control. (B) Effect of RAO on the involucrin promoter activity. Keratinocytes were transduced with 10 multiplicity of infections (MOIs) of involucrin-luc reporter adenovirus, then treated with RAO for 3 days. Cells were lysed and assayed for luciferase activity. Data are represented as relative light unit (RLU) and SEM, measured from 3 independent experiments. |
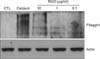 | Fig. 3Effect of rose absolute oil (RAO) on filaggrin expression. Normal human epidermal keratinocytes were treated with RAO at the indicated concentrations for 7 days. Calcium (1.2 mM) was included as a positive control for keratinocyte differentiation. Cellular proteins were prepared and protein level for filaggrin was evaluated by Western blot. Actin was used as a loading control. |
 | Fig. 4Effect of rose absolute oil (RAO) on the cell growth. Normal human epidermal keratinocytes were treated with 10µg/ml RAO for the indicated time points in the presence of 1µCi of [3H]thymidine. Calcium (1.2 mM) was included as a positive control for keratinocyte differentiation. Cells were lysed using 0.1 N NaOH and radioactivity was measured. Data are expressed as percentage of control. The mean values±SEM are averages of triplicate measurements. |
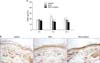 | Fig. 5Effect of rose absolute oil (RAO) on transepidermal water loss (TEWL). (A) Hairless mice were tape-stripped, then topically treated with 0.1% solution of RAO (in 70% polyethylene glycol and 30% ethanol) and RAO complex in which RAO (0.1%), oleic acid (0.1%), oleanolic acid (0.05%), urocanic acid (0.01%), acetylhexapeptide (0.01%) and ceramide (0.1%) were additionally included. At the indicated time points, TEWL was measured using Tewameter. Basal TEWL (0 h) was measured before tape-stripping. Five hairless mice were included in each group. Data are represented as average and SEM. (B) Skin specimens were obtained after 5 days of treatment. Sections were prepared and stained with anti-filaggrin antibody (×100). |
References
1. Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002. 24:789–800.

3. Blumenberg M, Tomić-Canić M. Human epidermal keratinocyte: keratinization processes. EXS. 1997. 78:1–29.

4. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.

5. Toulza E, Mattiuzzo NR, Galliano MF, Jonca N, Dossat C, Jacob D, et al. Large-scale identification of human genes implicated in epidermal barrier function. Genome Biol. 2007. 8:R107.

6. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995. 270:17702–17711.

7. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007. 445:866–873.

8. Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009. 124:1235–1244.

9. Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007. 8:1–12.

10. Lebwohl M, Herrmann LG. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis. 2005. 76:6 Suppl. 7–12.
12. Lynde C. Moisturizers for the treatment of inflammatory skin conditions. J Drugs Dermatol. 2008. 7:1038–1043.
13. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009. 84:1 Suppl. 2–15.
14. Kezic S, Kammeyer A, Calkoen F, Fluhr JW, Bos JD. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: evaluation of minimally invasive methods. Br J Dermatol. 2009. 161:1098–1104.

15. Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, et al. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008. 128:2117–2119.

16. Hwang C, Jang S, Choi DK, Kim S, Lee JH, Lee Y, et al. The role of Nkx2.5 in keratinocyte differentiation. Ann Dermatol. 2009. 21:376–381.

17. Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, α β-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009. 54:6–11.

18. Yoshizawa Y, Tanojo H, Kim SJ, Maibach HI. Sea water or its components alter experimental irritant dermatitis in man. Skin Res Technol. 2001. 7:36–39.

19. Seo EY, Namkung JH, Lee KM, Lee WH, Im M, Kee SH, et al. Analysis of calcium-inducible genes in keratinocytes using suppression subtractive hybridization and cDNA microarray. Genomics. 2005. 86:528–538.

20. Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001. 114(Pt 17):3069–3070.

21. Steinert PM, Marekov LN. Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope. J Biol Chem. 1997. 272:2021–2030.

22. Robinson NA, Lapic S, Welter JF, Eckert RL. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J Biol Chem. 1997. 272:12035–12046.

23. Chu DH. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Development and structure of skin. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;57–73.
24. Contet-Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol. 1999. 140:1038–1047.

25. Elias PM, Menon G, Wetzel BK, Williams JJ. Evidence that stress to the epidermal barrier influenced the development of pigmentation in humans. Pigment Cell Melanoma Res. 2009. 22:420–434.

26. Kurokawa I, Mizutani H, Kusumoto K, Nishijima S, Tsujita-Kyutoku M, Shikata N, et al. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen. 2006. 14:38–45.

27. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008. 17:1063–1072.

28. Ruether A, Stoll M, Schwarz T, Schreiber S, Fölster-Holst R. Filaggrin loss-of-function variant contributes to atopic dermatitis risk in the population of Northern Germany. Br J Dermatol. 2006. 155:1093–1094.

29. Proksch E, Fölster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006. 43:159–169.

30. Ulusoy S, Boşgelmez-Tinaz G, Secilmis-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr Microbiol. 2009. 59:554–558.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download