Abstract
Myoblast fusion depends on mitochondrial integrity and intracellular Ca2+ signaling regulated by various ion channels. In this study, we investigated the ionic currents associated with [Ca2+]i regulation in normal and mitochondrial DNA-depleted (ρ0) L6 myoblasts. The ρ0 myoblasts showed impaired myotube formation. The inwardly rectifying K+ current (IKir) was largely decreased with reduced expression of KIR2.1, whereas the voltage-operated Ca2+ channel and Ca2+-activated K+ channel currents were intact. Sustained inhibition of mitochondrial electron transport by antimycin A treatment (24 h) also decreased the IKir. The ρ0 myoblasts showed depolarized resting membrane potential and higher basal [Ca2+]i. Our results demonstrated the specific downregulation of IKir by dysfunctional mitochondria. The resultant depolarization and altered Ca2+ signaling might be associated with impaired myoblast fusion in ρ0 myoblasts.
Mononucleated myoblasts are differentiated into multinucleated myofibers through processes including cell fusion. The myoblast differentiation (myogenesis) is a critical process of skeletal muscle formation and regeneration after muscle damage [12]. During myogenesis, intracellular Ca2+ signaling is known to be critical for the fusion of myoblasts [3]. Appropriate and timely increase of intracellular Ca2+ concentration ([Ca2+]i) activates several myogenic transcription factors, including myogenin, myocyte enhancer factor-2 (MEF2), and nuclear factor of activated T-cells cytoplasmic 3 (NFATc3) [456].
Ca2+ channels are vital for maintaining elevated [Ca2+]i. In myoblasts, both Ca2+ release-activated Ca2+ (CRAC) channels and voltage-operated Ca2+ channels (VOCCs) contribute to the Ca2+ signal [789]. Among the types of VOCCs, the T-type Ca2+ channel is considered important for myoblast fusion [89]. While VOCCs are activated by membrane depolarization, CRAC channels are voltage-independent channels and require membrane hyperpolarization to maintain the electromotive force for Ca2+ influx. Therefore, the functional levels of K+ channels would differentially affect [Ca2+]i depending on the context of myogenesis signals; the depolarized resting membrane potential (RMP) would allow more Ca2+ influx as the membrane potential enters the window range of T-type Ca2+ channels. Myoblasts have been reported to express intermediate-conductance Ca2+-activated K+ channels (IKCa) and inwardly-rectifying K+ channels (Kir) [8101112].
The mitochondria generally produce most of the ATP in cells, and contribute to the regulation of intracellular Ca2+ signaling [13]. Previous studies demonstrated that mitochondrial DNA (mtDNA)-depleted (ρ0) myoblasts or myoblasts treated with mitochondrial metabolic inhibitors show higher [Ca2+]i levels than untreated myoblasts, even in the basal state [1415]. This phenomenon could be due to multiple factors such as reduced Ca2+ storing capability of endoplasmic reticulum (ER), decreased mitochondrial Ca2+ uptake, and attenuated Ca2+ removal via the plasma membrane [15]. Apart from the lack of energy because of mitochondrial depletion, changes in the abovementioned ion channel functions might also lead to the abnormal Ca2+ influx in the ρ0 myoblasts. However, no previous study has directly investigated the electrophysiological changes in ρ0 myoblasts.
In this study, we examined the effect of prolonged mitochondrial dysfunction on the ionic currents through VOCC (IVOCC), Ca2+-activated IKCa, and Kir (IKir) using the whole-cell patch clamp technique in control and ρ0 myoblasts. Our results demonstrate, first the first time, selective suppression of Kir and depolarized RMP in the ρ0 myoblasts that fail to differentiate into myofibers.
L6 rat skeletal myocytes stably expressing GLUT4 (L6 myoblasts) were obtained from Karafast (Boston, MA, USA). The mtDNA-depleted L6 myoblasts were culture and generation using a method described in a previous report [16]. Briefly, for the quantification of mtDNA content, real-time PCR was routinely conducted to monitor the amounts of cytochrome oxidase I (COXI), COXII, and COXIV in DNA using a method described previously [16]. For myoblast differentiation, L6 myoblasts were transferred to differentiation medium containing 2% horse serum, 100 U/ml penicillin, and 100 µg/ml streptomycin (all from Life Technologies, Carlsbad, CA, USA) in MEM-α.
RNA extraction and cDNA synthesis were carried out as previously described [16]. Briefly, RNA was extracted from EtBr/uridine-treated or non-treated cells using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Then, 1 µg of RNA was primed with oligo (dT) 20 primer (Bioneer, Daejeon, Republic of Korea) and reverse-transcribed at 42℃ for 50 min and 72℃ for 15 min. Gene-specific primers and 2 µl cDNA were used for the PCR analysis using a 7500 real-time PCR system, with Power SYBR® Green PCR Master Mix (both from Applied Biosystems, Foster City, CA, USA). The primer sequence details are provided in Table 1.
Protein was isolated using RIPA buffer supplemented with protease inhibitor (both from Thermo Fisher Scientific). Then 40 µg protein was electrophoresed on an 8% polyacrylamide gel containing SDS and transferred onto a nitrocellulose membrane (Millipore, Billerica MA, USA), which was then blocked with 5% BSA in Tris-buffered saline plus Tween (TBST) for 1 h. The membrane was incubated with anti-KIR2.1 (1:600) and anti-β-actin (1:2000; both from Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies in 1% BSA or skim milk overnight. After washing with TBST, the blots were incubated with HRP-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature (22–25℃) and developed using Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Cells were transferred into a bath mounted on the stage of an inverted microscope (TE2000-S, Nikon, Tokyo, Japan). The bath (−0.15 ml) was superfused at 3 ml/min, and voltage-clamp experiments were performed at room temperature (22–25℃). Patch pipettes with a free-tip resistance of approximately 3.5 and 4 MΩ were used for whole-cell patch-clamp assays. To measure ionic currents, conventional whole-cell patch-clamp recordings were conducted using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). To determine the membrane potential, current-clamp experiments were carried out using a nystatin-perforated whole-cell configuration. For nystatin treatment, the nystatin stock was sonicated and diluted in a pipette solution to a final concentration of 60–70 µg/ml. Nystatin was freshly prepared on each day of the experiments. The recorded data were digitalized at 5 kHz and low-pass-filtered using a Digidata 1440A (Molecular Devices). Patch pipettes were fabricated from borosilicate glass (World Precision Instruments, Sarasota, FL, USA) using a five step programmable Flaming-Brown puller (Model P-97, Shutter Instruments, Novato, CA, USA). A pulled pipette was fabricated to facilitate giga-seal formation using a microforge (Narishige, Tokyo, Japan). pCLAMP software v9.2 and Digidata-1322A (Molecular Devices) were used to acquire data and apply command pulses. Voltage and current trace data were saved on a desktop computer and analyzed using Clampfit Software 10.4 (Molecular Devices), Prism 6.0 (GraphPad Software, La Jolla, CA, USA), and Origin 8.0 (MicroCal, Northampton, MA, USA).
[Ca2+]i was measured in HEPES-buffered physiological salt solution (PSS) containing 145 mM NaCl, 3.6 mM KCl, 10 mM HEPES, 1.3 mM CaCl2, 1 mM MgCl2, and 5 mM D-glucose (pH 7.4). Cells were harvested in a normal bath solution, loaded with fura-2 acetoxymethyl ester (fura-2, 5 µM, 30 min, 25℃), and washed once with fresh PSS. Fluorescence was monitored in a stirred quartz microcuvette (1 ml) in the temperature-controlled cell holder of a fluorescence spectrophotometer (Photon Technology Instrument, Birmingham, NJ, USA) at wavelengths of 340 and 380 nm (excitation) and 510 nm (emission). The calibration of Ca2+ concentrations was described in a previous report [17].
Normal Tyrode's bath solution for the whole-cell patch-clamp assay contained 145 mM NaCl, 3.6 mM KCl, 1 mM MgCl2, 1.3 mM CaCl2, 10 mM glucose, and 10 mM HEPES, at a pH of 7.4 (adjusted with NaOH). The high-K+ solution for whole-cell patch clamp contained 145 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 10 mM HEPES, at a pH of 7.4 (adjusted with NaOH). The pipette solution for whole-cell patch clamp contained 145 mM KCl, 3 mM MgCl2, 10 mM HEPES, 3 mM ATP magnesium salt, and 1 mM EGTA, at a pH of 7.2 (adjusted with KOH). The pipette solution of 1 µM free Ca2+ contained 135 mM KCl, 8.7 mM CaCl2, 1 mM MgCl2, 10 mM EGTA, 5 mM HEPES, and 3 mM ATP, at a pH of 7.2 (adjusted with KOH).
All results were analyzed using the GraphPad Prism 6.0 and Origin 8.0 software programs, and the data are expressed as the mean±standard error of the mean. Means of the control and ρ0 myoblast groups were compared using an unpaired t-test and a p<0.05 was considered statistically significant.
To confirm the mitochondrial reduction, we determined the relative amounts of mtDNA (COX1 and COX2) and nuclear DNA (COX4). The levels of COX1 and COX2 in ρ0 myoblasts were ≤ 20% of those in the control myoblasts, whereas the level of COX4 was unchanged (Fig. 1A). In addition, the total cellular ATP content of the ρ0 myoblasts was approximately 30% of that of the control myoblasts, indicating impaired mitochondrial function (Fig. 1B). Five days of culture in horse serum-free medium induced differentiation of control myoblasts, but not ρ0 cells, into myotubes (Fig. 1C).
To record the VOCC current, whole-cell patch clamp was conducted using a CsCl-rich pipette solution. Depolarizing step pulses from a holding voltage of −80 mV revealed transient inward currents from above −50 mV (Fig. 2A, inset). Plotting the peak amplitudes of IVOCC density (pA/pF) according to the test voltages revealed typical U-shape I/V curve of VOCC (Fig. 2A). The current densities of VOCC were not different between ρ0 and control cells; −35.5±7.44 pA/pF and −25.5±3.65 pA/pF at 0 mV in control and ρ0 myoblasts, respectively (Fig. 2B).
To record IKCa, KCl-rich pipette solution with increased Ca2+ activity (1 µM free Ca2+ clamped with 10 mM EGTA) was adopted. Ramp-like depolarization from −100 to 100 mV revealed voltage-independent large outward currents, consistent with the known property of IKCa (Fig. 2C). The amplitudes of IKCa density were quite variable, but not statistically different between control and ρ0 myoblasts; 208.8±34.92 pA/pF and 265.8±44.39 pA/pF at +25 mV, respectively (Fig. 2D).
To record the IKir, Ca2+-free KCl pipette solution and 145 mM KCl bath solution were used for the whole-cell patch clamp applying ramp-like pulse from −140 to 70 mV. Under similar KCl conditions, large inward K+ currents were recorded at the negative clamp voltage range, and the I/V curves showed typical inward rectification property (Fig. 3A). Interestingly, IKir was largely decreased in ρ0 myoblasts (Figs. 3A and B). The current densities were −50.6±15.81 pA/pF and −13.6±5.42 pA/pF at −120 mV in the control and ρ0 myoblasts, respectively. For the molecular identity of Kir, the KIR2.1 channel is known to be expressed in myoblasts [10] and the immunoblot analysis revealed that its expression level was decreased by 31±0.1% in the ρ0 cells.
To elucidate the mechanism underlying the IKir decrease in ρ0 myoblasts, normal myoblasts were treated with antimycin A (10 µM), a mitochondrial complex III inhibitor, for 24 h [18]. The density of IKir was also drastically decreased in the antimycin A-treated cells compared with that in untreated cells (Figs. 3D and E, −63.9±13.35 pA/pF vs. −2.6±2.08 pA/pF at −120 mV).
To measure the RMP of myoblasts under physiological conditions, we used a nystatin-perforated patch clamp that preserved cellular components other than intracellular monovalent cations [19]. Consistent with the decrease in IKir, the RMP of ρ0 myoblasts was more depolarized than that of the control; −63.7±3.06 mV vs. −47.9±3.6 mV (Fig. 4A). Moreover, in accordance with a previous study [15], the resting [Ca2+]i in ρ0 myoblasts was higher than that in control myoblasts was (146.2±8.74 nM vs. 114.1±5.31 nM, Fig. 4B).
We also assessed SOCE from the sustained increased in [Ca2+]i induced by thapsigargin (TG, 2 µM), which inhibits the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor. The TG-induced [Ca2+]i increase (Δ[Ca2+]i), a parameter reflecting steady-stated store-operated Ca2+ entry (SOCE), was slightly lower in ρ0 than it was in control myoblasts (Fig. 4C, Δ[Ca2+]i at 200 sec was 260.6±24.83 nM and 152±26.23 nM in the control and ρ0 myoblasts, respectively, p<0.05).
In this study, we found, for the first time, markedly decreased IKir along with altered [Ca2+]i and SOCE in mtDNA-depleted myoblasts. Since the ρ0 myoblasts did not differentiate into myotubes, the results suggest that Kir might be a myogenesis-related ion-channel that regulates intracellular Ca2+ signaling, although indirectly.
Since Ca2+ influx through the VOCC is known to be critical for the fusion of myoblasts [9], we initially anticipated a decrease in IVOCC in the ρ0 myoblasts, however, this was not the case. In excitable cells, the activation of IKCa facilitates repolarization when the [Ca2+]i is above the threshold level. In the ρ0 myoblasts, IKCa density at the fixed concentration was not altered. Although more experiments are warranted, the results further emphasize the effects of the dramatic decrease in IKir. It has been reported that the ether-a-go-go-related gene (ERG) channel, a member of the voltage-gated K+ channel family is expressed in myoblasts [820]. However, we did not observe significant levels of the putative ERG channel current in L6 myoblasts (data not shown).
Because IKir is important in setting the RMP in many types of excitable cells, the dramatic decrease in IKir and the downregulation of KIR2.1 in ρ0 myoblasts were interesting findings. However, compared to the functional decrease of IKir, the immunoblot assays showed that the decrease in KIR2.1 expression of the total cell lysate was relatively less significant (Fig. 3). More specific assays of KIR2.1 in the plasma membrane might facilitate a more precise correlation between the IKir protein expression and function.
Interestingly, pharmacological inhibition of the mitochondrial electron transport chain (ETC) complex III by antimycin A also induced similar decrease in the IKir. Although it is unclear how functional inhibition of the mitochondria selectively decreased the IKir in myoblasts, the effects of antimycin A treatment also suggested that sustained inhibition of oxidative phosphorylation, i.e., ATP depletion, appeared critical for maintaining KIR2.1 expression. The KIR2.1 activity is well known to be sensitively regulated by membrane phosphatidylinositol(4,5)-bisphosphate (PIP2) [2122]. Since the production of PIP2 by PI kinase requires ATP, the reduced intracellular ATP induced by mtDNA depletion or antimycin treatment might have decreased PIP2 in the cell membranes, thereby reducing the activity and functions of KIR2.1.
Recently, it was reported that activation of AMPK, a critical intracellular metabolic sensor with various signaling functions, may be implicated in the downregulation of KIR2.1. Particularly, the activation of AMPK by ATP-depletion or increase in the cytosolic Ca2+ activity decreases KIR2.1 abundance in the cell membrane of Xenopus oocytes through the activation of the AMPK by activating the ubiquitin ligase Nedd4-2 [23]. Recently, Zhao et al. [24] reported that mitochondrial dysfunction induced by genetic defects or mitochondria-targeted antioxidants activates AMPK signaling in human keratinocytes. Therefore, activation of AMPK might be one of the mechanisms underlying the IKir decay in the present study [24]. In agreement with previous results [24], we also observed increased expression and phosphorylation of AMPK in the mtDNA-depleted myocytes in this study (data not shown). Therefore, further studies would be essential to elucidate the exact mechanisms underlying the inhibition of KIR2.1 activity by prolonged mitochondrial inhibition.
Although proper Ca2+ signaling is known to be required for the differentiation of myoblasts, the abnormal or untimely increase in basal [Ca2+]i might have hampered the differentiation of the ρ0 myoblasts in the present study. Considering the robust activity of VOCC, the higher resting [Ca2+]i might, at least partly, be due to the IKir decrease and membrane depolarization, as partial depolarization of the RMP would increase the Ca2+ influx via VOCCs [2526]. Another plausible mechanism underlying the resting [Ca2+]i increase could be impaired ATP supply to the various active Ca2+ transporters such as SERCA, which should also be considered for proper interpretation of the findings.
It has also been suggested that the so-called Ca2+ release-activated Ca2+ (CRAC) channel is important for myogenesis [7]. Since CRAC channels are mainly responsible for the Ca2+ influx pathway of SOCE, the lower SOCE in ρ0 myoblasts also attracted our attention. However, since we did not directly measure the electrical Ca2+ current through CRAC channels in L6 myoblasts, the impli cation of partial decrease in the thapsigargin-induced Δ[Ca2+]i could not be provided here. Since the maximum levels of [Ca2+]i were not significantly different between the control and ρ0 myoblasts (392.28±2.45 nM and 328.13±21.94, respectively, p>0.05, Fig. 4C), we cautiously suggest that the decrease in IKir and partial depolarization might be partly responsible for the lower SOCE in ρ0 myoblasts induced by reducing the electromotive driving force of the Ca2+ influx. Furthermore, the higher basal [Ca2+]i would have inactivated the CRAC channels [27]. To avoid the complexity of Ca2+-induced inactivation of CRAC channels, we measured the SOCE using the Ca2+-add back protocol (i.e., thapsigargin was applied after incubating the cells under Ca2+-free conditions, and then Ca2+ was added to the bath), which would more precisely reflect the maximum SOCE. The exact implication of the higher basal [Ca2+]i with lower SOCE in ρ0 myoblasts would also require further studies using patch clamp and a more rigorous protocol for SOCE measurement.
Figures and Tables
Fig. 1
Characterization of mtDNA-depleted L6 GLUT4myc myocytes.
(A) Comparison of cytochrome oxidase I (COX1), COX2, and COX4 levels between normal L6 GLUT4myc myocytes (control) and mtDNA-depleted L6 GLUT4myc myocytes (ρ0 myoblasts) using real-time PCR. Relative intensities are presented as normalized values with the intensity of control set to 1. ****p<0.0001. (B) Comparison of total cellular ATP level between control and ρ0 myoblasts. ATP contents expressed as normalized value, which was set to 1. ****p<0.0001. (C) Comparison of differentiation of myoblasts into myotubes between control and ρ0 myoblasts.
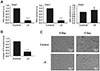
Fig. 2
Voltage-operated Ca2+ channel (VOCC) and Ca2+ activated K+ channel are not affected by mtDNA depletion.
(A) Representative current traces of myocyte responses to each test pulse (inset). For whole-cell patch-clamp recordings, a test pulse was applied to myocytes for 500 ms, and voltage pulses were applied from the holding potential (−80 mV) in decrements or increments of Δ10 mV between −100 mV and 100 mV. Average current (I)–voltage (V) relationship curve for the peak VOCC current at each voltage in response to each test pulse between control (n=12) and ρ0 myoblasts (n=14). (B) Summary of the current density of VOCC averaged at 0 mV. All data are means±standard error of the mean. (C) Average I–V relationship curve in control and ρ0 myoblasts in response to 1 µM [Ca2+]i pipette solution. (D) Summary of Ca2+-activated K+ current (IKCa) at +25 mV in control and ρ0 myoblasts.

Fig. 3
Downregulation of inward-rectifying K+ channel in ρ0 myoblasts.
(A) Representative chart trace recordings of inward-rectifying K+ current (IKir) in symmetrical KCl solution. For whole-cell patch-clamp recordings, the current was obtained by step depolarizing pulse ranging from −140 mV to 70 mV at a holding potential of 0 mV. (B) Average I–V relationship curve for the peak IKir from −140 to 70 mV between control (n=13) and ρ0 myoblasts (n=13). *p<0.05. (C) Immunoblot assay of KIR2.1 and β-actin expression in control and ρ0 myoblasts. KIR2.1 signals were normalized to the β-actin signal, and mean values are displayed as bar graphs (n=3). *p<0.05. Data are means±standard error of the mean. (D) Average I–V relationship curve of IKir following treatment with antimycin A (n=7) and in control myoblasts (n=7) in normal myocytes. (E) Current density of IKir at −120 mV. **p<0.01. All data are presented as the mean±standard error of the mean.

Fig. 4
Resting membrane potential (RMP) is depolarized and store-operated Ca2+ entry (SOCE) is diminished in ρ0 myoblasts.
(A) Comparison of RMP between control (n=8) and ρ0 myoblasts (n=12). (B) Average [Ca2+]i between control (n=13) and ρ0 myoblasts (n=6) in the resting state. (C) Average trace recordings of [Ca2+]i, which increased following thapsigargin (2 µM) treatment of control (black) and ρ0 (gray) myoblasts. Data are means±standard error of the mean (n=3). **p<0.01.

ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT and Future Planning (MIST) of the Korean government (grant number NRF-2015R1C1A1A02037738).
Notes
Author contributions S.J.K., W.L., and J.H.N. conceived and supervised the study; J.H.W., H.J.K., E.J.L., and I.C. designed the experiments; J.H.W., H.J.K., and E.J.L. performed the experiments; J.H.W., H.J.K., E.J.L., I.C., S.J.K., Y.K.K., W.L., and J.H.N. analyzed and interpreted the data; and S.J.K., W.L. and J.H.N. wrote the manuscript.
References
1. Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012; 139:641–656.

3. David JD, See WM, Higginbotham CA. Fusion of chick embryo skeletal myoblasts: role of calcium influx preceding membrane union. Dev Biol. 1981; 82:297–307.

4. Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998; 14:167–196.

5. Friday BB, Pavlath GK. A calcineurin- and NFAT-dependent pathway regulates Myf5 gene expression in skeletal muscle reserve cells. J Cell Sci. 2001; 114:303–310.

6. Molkentin JD, Olson EN. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc Natl Acad Sci U S A. 1996; 93:9366–9373.

7. Darbellay B, Arnaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009; 284:5370–5380.

9. Bijlenga P, Liu JH, Espinos E, Haenggeli CA, Fischer-Lougheed J, Bader CR, Bernheim L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc Natl Acad Sci U S A. 2000; 97:7627–7632.
10. Fischer-Lougheed J, Liu JH, Espinos E, Mordasini D, Bader CR, Belin D, Bernheim L. Human myoblast fusion requires expression of functional inward rectifier Kir2.1 channels. J Cell Biol. 2001; 153:677–686.

11. Konig S, Béguet A, Bader CR, Bernheim L. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006; 133:3107–3114.
12. Tanaka S, Ono Y, Sakamoto K. DCEBIO facilitates myogenic differentiation via intermediate conductance Ca2+ activated K+ channel activation in C2C12 myoblasts. J Pharmacol Sci. 2017; 133:276–279.
14. Wagatsuma A, Sakuma K. Mitochondria as a potential regulator of myogenesis. ScientificWorldJournal. 2013; 2013:593267.

15. Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of interorganelle crosstalk. EMBO J. 1999; 18:522–533.
16. Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Depletion of mitochondrial DNA causes impaired glucose utilization and insulin resistance in L6 GLUT4myc myocytes. J Biol Chem. 2005; 280:9855–9864.

17. Nam JH, Lee DU. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J Dermatol Sci. 2016; 84:305–313.

18. Ma X, Jin M, Cai Y, Xia H, Long K, Liu J, Yu Q, Yuan J. Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem Biol. 2011; 18:1474–1481.

19. Okada Y. Patch clamp techniques: from beginning to advanced protocols. Tokyo: Springer;2012.
20. Bernheim L, Bader CR. Human myoblast differentiation: Ca2+ channels are activated by K+ channels. News Physiol Sci. 2002; 17:22–26.
21. D'Avanzo N, Cheng WW, Doyle DA, Nichols CG. Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2010; 285:37129–37132.
22. Kim KS, Jang JH, Lin H, Choi SW, Kim HR, Shin DH, Nam JH, Zhang YH, Kim SJ. Rise and fall of Kir2.2 current by TLR4 signaling in human monocytes: PKC-dependent trafficking and PI3K-mediated PIP2 decrease. J Immunol. 2015; 195:3345–3354.
23. Alesutan I, Munoz C, Sopjani M, Dërmaku-Sopjani M, Michael D, Fraser S, Kemp BE, Seebohm G, Föller M, Lang F. Inhibition of Kir2.1 (KCNJ2) by the AMP-activated protein kinase. Biochem Biophys Res Commun. 2011; 408:505–510.

24. Zhao B, Qiang L, Joseph J, Kalyanaraman B, Viollet B, He YY. Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes Dis. 2016; 3:82–87.

25. Engbers JD, Anderson D, Zamponi GW, Turner RW. Signal processing by T-type calcium channel interactions in the cerebellum. Front Cell Neurosci. 2013; 7:230.

26. Lee N, Jeong S, Kim KC, Kim JA, Park JY, Kang HW, Perez-Reyes E, Lee JH. Ca2+ egulation of Cav3.3 T-type Ca2+ channel is mediated by calmodulin. Mol Pharmacol. 2017; 92:347–357.
27. Scrimgeour NR, Wilson DP, Barritt GJ, Rychkov GY. Structural and stoichiometric determinants of Ca2+ release-activated Ca2+ (CRAC) channel Ca2+-dependent inactivation. Biochim Biophys Acta. 2014; 1838:1281–1287.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download