Abstract
A beneficial radioprotective agent has been used to treat the radiation-induced lung injury. This study was performed to investigate whether curcumin, which is known to have anti-inflammatory and antioxidant properties, could ameliorate radiation-induced pulmonary inflammation and fibrosis in irradiated lungs. Rats were given daily doses of intragastric curcumin (200 mg/kg) prior to a single irradiation and for 8 weeks after radiation. Histopathologic findings demonstrated that macrophage accumulation, interstitial edema, alveolar septal thickness, perivascular fibrosis, and collapse in radiation-treated lungs were inhibited by curcumin administration. Radiation-induced transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF) expression, and collagen accumulation were also inhibited by curcumin. Moreover, western blot analysis revealed that curcumin lowered radiation-induced increases of tumor necrosis factor-α (TNF-α), TNF receptor 1 (TNFR1), and cyclooxygenase-2 (COX-2). Curcumin also inhibited the nuclear translocation of nuclear factor-κ B (NF-κB) p65 in radiation-treated lungs. These results indicate that long-term curcumin administration may reduce lung inflammation and fibrosis caused by radiation treatment.
Radiation therapy is widely used to treat inoperable solid tumors and hematologic malignancies in conjunction with chemotherapy [1,2]. Many studies have described ways to prevent or ameliorate radiation-induced lung injury [3-6]. These include applying the minimum dosage of radiation, minimizing the treatment field to prevent normal lung being included in the therapeutic irradiation zone, and using a radioprotective agent.
Although the molecular mechanisms of radiation-induced lung injury pathogenesis are not fully understood, many researchers insist that the cytokine transforming growth factor-β1 (TGF-β1) is an important component [7,8]. One study demonstrated the potent fibrinogenic action of TGF-β1 in damaged lung tissue after thoracic irradiation and showed that it led to lung fibrosis [9]. Anscher et al. [10] found that the blocking the TGF-β1 pathway with a specific antibody ameliorated radiation-induced lung injury in rats.
Tumor necrosis factor-α (TNF-α) is another important cytokine involved in radiation-induced lung injury. TNF-α-mediated nuclear factor kappa-B (NF-κB) activation enhances the release and production of pro-inflammatory cytokines and cyclooxygenase-2 (COX-2) [11]. In an animal study, TNF-α-mediated activation of NF-κB was shown to enhance COX-2 expression in thoracic irradiation-damaged lung tissue [12].
Curcumin exerts potent anti-inflammatory effects by inhibiting pro-inflammatory cytokines and modulating adhesion molecules [13,14]. These suppressive effects are achieved through the inhibition of NF-κB-mediated inflammation [15-18]. Previous evidence showed that oral curcumin administration reduced the lung collagen hydroxyproline in rats with lung fibrosis induced by whole-body irradiation [19]. Recently, several studies described antioxidant molecules as potential radioprotective agents of curcumin including flaxseeds and omega-3 [20,21]. The intake of dietary curcumin after thoracic irradiation attenuates pulmonary fibrosis, inflammation and oxidative lung damage and also improves survival in Lewis lung carcinoma cell injected mice [22].
In the present study, we evaluated the effect of curcumin on radiation-induced pulmonary inflammation and collagen-associated fibrosis in rat lungs 8 weeks after radiation.
Male Sprague Dawley rats (n=32) initially weighing 250~270 g were purchased from Japan SLC (Hamamatsu, Japan) and maintained in the animal facility of the Gyeongsang National University School of Medicine. The study was approved by institutional Board of Research of the Gyeongsang National University and performed in accordance with standard guidelines for laboratory animal care. Rats were housed three per cage and allowed to acclimate for 1 wk prior to treatment. The cages were kept in rooms with an alternating 12-h light/dark cycle, and they received standard rodent chow and water ad libitum. Rats were randomly divided into four groups: control (CTL, n=5), irradiated (RT, n=11), irradiated along with curcumin (RT+Cur, n=11), and curcumin only (Cur, n=5).
Rats were anesthetized with an intramuscular injection of 2% xylazine hydroxychloride (3 mg/kg, Rumpun; Bayer Korea Ltd., Seoul, Korea) and tiletamine-zolazepam (2 mg/kg, Zoletil 50; Virbac Laboratory, Carros, France) prior to radiation exposure. Rats received a single 18-Gy dose of thoracic irradiation using a 4 MV linear accelerator (21EX 3153 VARIAN, Palo Alto, CA, USA). Rats were positioned so that the entire thoracic cavity (including both lungs) was within the radiation field. A 3-cm block of Lucite was positioned above the chest to provide adequate buildup and facilitate even radiation distribution. Curcumin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and mixed with 0.5% carboxylmethylcelluose sodium salt in 0.9% normal saline. According to previous studies [23-25], rats were given intragastric administrations of curcumin. Beginning one wk before radiation exposure until the end of the study (9 wk), RT+Cur and Cur rats received curcumin (200 mg/kg) five times per wk.
All animals were sacrificed 8 wks after thoracic irradiation. A median thoracotomy was performed under general anesthesia with zoletil (5 mg/kg, Virbac Laboratories, Carros, France), and a blood sample was obtained by direct ventricular puncture to measure serum TGF-β1 concentration. For western blot analysis, the right lung was extracted and stored at -80℃ until use. The left lung was removed for histological examination.
Tissue samples were fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), embedded in paraffin, and 5-µm sections were cut. To assess the degree of inflammation and fibrosis in each group, sections were stained with hematoxylin and eosin (H&E) and Masson's trichrome, respectively. We evaluated macrophage accumulation, interstitial edema, alveolar septal thickening, and perivascular fibrosis, which were scored on a scale of 0 (absent) to 4 (severe) (Score 0: -, Score 1: +/-, Score 2: +, Score 3: ++, Score 4: +++), as described previously [26]. Each slide received four scores for separate fields, and the mean value was considered representative of the slide. Alveolar septa thickness was assessed by measuring the length of a straight line that crossed the alveolar wall with Image-Pro Plus software (Media Cybernectics, Bucking-hamshire, UK). The mean thickness was obtained for four measurements from each slide; the means for each animal were used to calculate the average alveolar septal thickness of each group.
The Sircol collagen assay is a dye-binding method used to analyze newly synthesized acid- and pepsin-soluble collagens during inflammation and wound healing. The lung tissues (n=5~6 per group) had been previously frozen in liquid nitrogen and stored at -80℃ until assayed. The samples were thawed, and collagen concentration was analyzed using the Sircol assay kit (Biocolor Ltd., Northern Ireland, UK) according to the manufacturer's instructions. A standard curve was used to calculate the collagen content of each sample.
Serum concentrations of TGF-β1 (n=5~6 per group) were quantitatively measured using a TGF-β1 rat enzyme-linked immunosorbent assay (ELISA) kit (Alpco Diagnostics, Salem, NH, USA) according to the manufacturer's instructions.
After deparaffinization, sections were sequentially treated with 1% H2O2 for 10 min and rinsed thoroughly with PBS. Sections were blocked with 2% normal goat serum in PBS at room temperature for 60 min to minimize non-specific immunoglobulin G (IgG) binding, followed by incubation with rabbit anti-F4/80 (dilution 1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-NF-κB p65 (dilution 1:100; Santa Cruz Biotechnology) or rabbit anti-COX-2 (dilution 1:100; Cayman Chemical, Ann Arbor, MI, USA) antibodies. The samples were washed with PBS, incubated for 60 min at room temperature with biotin-conjugated secondary IgG (diluted 1:200; Vector Laboratories, Burlingame, CA, USA), diluted in 2% normal blocking serum, and then incubated for 60 min at room temperature with avidin-biotin-peroxidase complex (ABC Elite kit, Vector Laboratories). The samples were then washed with PBS and incubated for 3 min with diaminobenzidine tetrahydrochloride (DAB, Sigma) containing 0.05% hydrogen peroxidase to develop color. The sections were visualized under light microscopy (model BX51, Olympus, Tokyo, Japan), and digital images were captured and analyzed. For each treatment group, F4/80-positive cells were manually counted in three sections of each rat (n=5~6 per group). The F4/80 positive cells were counted by observers blinded to the treatment conditions using 40× objectives.
Total extraczts and nuclear fractions were prepared from the right lung according to the protocol described by Müller et al. [27]. Lysate protein concentration was determined using bovine serum albumin (BSA) standards and a bicinchoninic acid (BCA) kit (Pierce/Thermo Scientific, Rockford, IL, USA). Equal amounts of protein (30 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were washed in Tris-buffered saline containing 0.5% Tween-20 (TBST) and incubated with the following TBST-diluted primary antibodies: rabbit anti-TGF-β1 (Chemicon International/Millipore, Temecula, CA, USA); rabbit anti-TNF-α, mouse anti-TNFR1, rabbit anti-TNFR2, and rabbit anti-NF-κB p65 (Santa Cruz Biotechnology); rabbit anti-COX-2 (Cayman); and rabbit anti-connective tissue growth factor (CTGF, Abcam, Cambridge, UK); rabbit anti-acetylated NF-κB p65 (Abcam). The samples were then incubated with their corresponding secondary antibodies. An enhanced chemiluminescence (ECL) western blot analysis system (Amersham Pharmacia Biotech/GE Healthcare, Piscataway, NJ, USA) was used for detection. Band densities were normalized to α-tubulin expression levels. β-actin and lamin A were used as internal control for cytosolic and nuclear extracts, respectively.
All statistical analyses were performed using statistical package for social sciences (SPSS, version 12.0, SPSS inc., Chicago, IL, USA). Results are presented as means±standard error of mean (SEM). Differences between groups were analyzed using one-way analysis of variance (ANOVA), followed by Student Newman-Keul tests for pairwise multiple comparisons. Results were considered statistically significant if p<0.05.
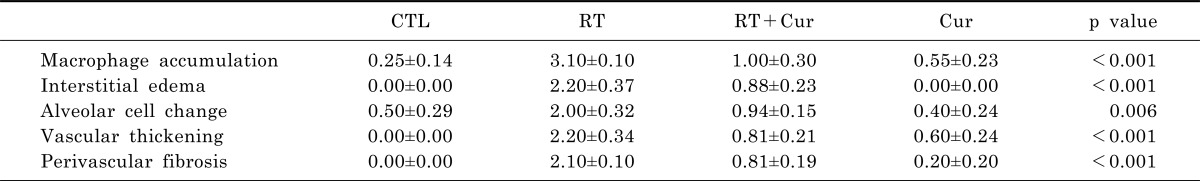
We hypothesized that curcumin exerts an anti-inflammatory effect in irradiated rat lung. To assess the effect of curcumin on radiation-induced inflammation, rats were pretreated with oral curcumin at 200 mg/kg/day from day-7 to the end of study and exposed to single irradiation. Eight weeks after radiation exposure, many macrophages had infiltrated rat lung alveoli. Inflammation was assessed in H&E-stained lung sections (Fig. 1A). We observed marked interstitial edema and macrophage accumulation in lung tissue from irradiated rats but not controls (Table 1). However, both pathologies were inhibited by curcumin treatment. To confirm these findings, we performed an immunohistochemistry for the macrophage marker F4/80 (Fig. 1B). F4/80-immunostained macrophage cells were observed in lung sections obtained from both irradiated groups. Importantly, F4/80-positive macrophages were significantly increased in radiation-treated lungs (30.94±1.68) compared to controls (4.87±0.42, p<0.001) and significantly decreased in radiation-treated lungs with curcumin (11.00±0.80) compared to the radiation-treated lungs (p<0.001).
We measured alveolar septal thickness in H & E-stained lung sections (Fig. 2). We observed that radiation-induced pneumonitis caused alveolar inflammation and remodeling (Table 1, Fig. 2A). However, curcumin significantly attenuated the increased alveolar septal thickness that occurred following radiation (Fig. 2B). Next, we performed Masson's trichrome staining and Sircol collagen assay (Fig. 2C and D) to determine whether curcumin effectively inhibited radiation-induced collagen accumulation and fibrosis. As shown in Figure 2C, vascular thickening and perivascular fibrosis were increased in irradiated lungs. However, curcumin attenuated both pathological changes (Table 1). This finding was confirmed by the results of the Sircol collagen assay (Fig. 2D), which showed that increased collagen content in irradiated lungs was ameliorated in curcumin-treated rats.
To evaluate whether curcumin could attenuate radiation-induced collagen synthesis in rat lungs, we assessed serum TGF-β1 levels by ELISA and TGF-β1 and CTGF expression levels with western blots (Fig. 3). Serum TGF-β1 levels from irradiated rat lungs were significantly increased compared to irradiated curcumin-treated rats (Fig. 3A). Western blots revealed that radiation-induced TGF-β1 and CTGF expression were reduced by curcumin (Fig. 3B and C).
The western blots in Fig. 4A illustrate that curcumin attenuated increased TNF-α and TNFR1 expression levels in radiation-induced lungs. However, neither radiation nor curcumin affected TNFR2 expression in rat lungs.
First, we performed western blots and determined that curcumin effectively inhibited NF-κB p65 nuclear translocation in irradiated lungs (Fig. 5A). Correspondingly, we found that radiation-induced increased NF-κB p65 immunoreactivity was decreased by curcumin (Fig. 5B). In addition, in comparison with controls, acetylated p65 NF-κB was increased in irradiated lungs and its acetylation was inhibited by curcumin treatment (Fig. 5C and D). Finally, western blot analysis and immunohistochemical experiments demonstrated that curcumin also inhibited radiation-induced COX-2 expression (Fig. 6).
Radiation-induced lung injury is characterized by pulmonary inflammation and alveolar fibrosis. Although it has been reported that dietary curcumin has potential therapeutic effects due to its anti-inflammatory effects, the TGF-β1-mediated fibrotic mechanisms of curcumin in radiation-induced lung injury were unclear. In the present study, we demonstrated that long-term curcumin administration attenuates radiation-induced inflammation and collagen-mediated fibrosis in rat lung. Specifically, curcumin reduces collagen levels and CTGF expression in irradiated lung. These findings suggest that curcumin may play an important role in inhibiting NF-κB-mediated inflammation and TGF-β1-mediated fibrotic lung remodeling downstream of irradiation injury.
Radiation-induced lung injury is also marked by macrophage infiltration, interstitial edema, and subsequent fibrotic changes including alveolar wall thickening and perivascular fibrosis [28,29]. Several studies have suggested that radiation-induced lung inflammation is related to the pneumonitis phase [30,31]. During late-phase pneumonitis after radiation, the chronic fibrosis period begins and is characterized by progressive alveolar septa fibrosis, and collagen accumulates in the interstitium and perivascular areas, leading to alveolar septal thickening [32,33]. The two responses of both pneumonitis and fibrosis period are not completely separate 8 wks after radiation exposure, and partly overlap. The present study started from the hypothesis that curcumin inhibits TGF-β1, COX-2, and NF-κB, which are the primary mediators related to inflammation, as this finding could give rise to protective or ameliorative effects of curcumin on radiation-induced pneumonitis. Thus, we examined the inflammation-lowering effects of curcumin at the climax of inflammation and to examine the effects of curcumin in the initial stage of fibrosis progression in only one time point 8 wks after radiation exposure.
In the present study, curcumin effectively inhibited radiation-induced lung injuries. Our findings are consistent with those of a study demonstrating that cyclo-(L-Phenylalanyl-L-Prolyl) or adenoviral vector expressing a soluble TGF-β receptor (AdTβ-ExR) ameliorates fibroproliferative changes in the lung at 8 wks postirradiation [34]. Previous reports have shown that supplemental dietary vitamin A inhibits radiation-induced lung fibrosis [5], and oral curcumin diminishes radiation-induced alveolitis in rat models [4]. Our data further indicate that long-term curcumin administration attenuates radiation-induced inflammation and fibrotic changes in rat lung during the pneumonitis phase following irradiation.
One of the most important pieces of evidence from the current study is that macrophage accumulation is significantly decreased by curcumin. It has been reported that the number of macrophages increases shortly after irradiation, and they subsequently produce a wide spectrum of cytokines that regulate fibroblast proliferation and the production of extracellular matrix components, such as TNF-α, TGF-β1, platelet-derived growth factor (PDGF), and interleukin-4 [35]. These inflammatory cytokines have been associated to pulmonary fibrosis because of their ability to regulate matrix production [36]. These cytokines induce collagen synthesis and deposition and also recruit other types of inflammatory cells that cause more extreme inflammation and fibrosis in damaged lung tissue [37-39]. In particular, the role of TNF-α in the development of fibrosis has been extensively investigated. TGF-β1 is also a key cytokine in the fibrotic process and radiation-induced lung fibrosis [40]. In accordance with our study, Park et al reported that TGF-β1 was increased from 4~8 weeks after 20 Gy of radiation [41]. In this point, we hypothesized that the cellular mechanism associated with the fibrotic process is due to repeated episodes of acute inflammation leading to excessive healing characterized by collagen accumulation and extracellular matrix proteins. Two responses of both acute injury and fibrosis were simultaneously appeared in the rat lungs 8 weeks after irradiation. In the present study, we supported the evidence that the TNF-α protein expression level in the RT group appears to be very strong. First, fibrotic cytokines such as TGF-β1 and CTGF were increased in radiation-treated rat lungs. Secondly, we found that increased nuclear translocation of NF-κB in irradiated lungs was reduced by curcumin. Therefore, we suggest that proinflammatory and fibrotic cytokines such as TNF-α, TGF-β1, and CTGF play an important role in radiation-induced lung injury.
It was recently reported that irradiated patients with locally advanced lung cancer who received ambroxol treatment exhibited a statistically significant decrease in serum TGF-β1 compared to a control group [42]. Our results support the concept that serum TGF-β1 is a useful parameter for evaluating treatment effects in patients with radiation-induced lung injuries. Furthermore, we found that nuclear NF-κB p65 levels decreased in response to curcumin treatment, indicating that curcumin blocked the radiation-induced activation of NF-κB. We also confirmed that the increase of NF-κB p65 acetylation indicates hyperactive NF-κB signaling in irradiated rat lungs. Curcumin suppressed the radiation-induced expression of NF-κB-regulated COX-2. Our results are consistent with a recent report showing that curcumin inhibits radiation-induced NF-κB activation by suppressing IκBα activation in human colorectal cancer cells [43]. With regard to pulmonary fibrosis, COX-2 has been shown to be important regulator of lung diseases [44]. Radiation-induced COX-2 expression was attenuated by COX-2 inhibitor [45]. Although disruption of COX-2 activity worsens bleomycin-induced lung dysfunction, COX-2 deficiency did not alter the extent or severity of fibrosis [46]. These data indicate that curcumin inhibits radiation-induced COX-2 expression by inhibiting NF-κB nuclear translocation and the other COX-2-independent mechanisms may be contributed to pulmonary fibrosis.
In the present study, there are some limitations. First, we did not explore the dose-dependent effects of curcumin on radiation-induced lung injury. Previous studies showed that 25, 50, 100, 150, or 200 mg/kg of oral curcumin supplementation in the excision wound model after radiation exposure showed dose-dependent elevation in the wound contraction [25]. Furthermore, curcumin (200 mg/kg) had protective effects against nicotine-induced lung injury or radiation-induced acute oral mucositis in the rats [23,24]. Based on previous studies, the daily dose (200 mg/kg) of curcumin was performed for the prevention of radiation-induced lung injury for 8 wks in this study. In fact, the relationships between supplemental doses and therapeutic doses have not been clearly defined. Thus, to know the optimal therapeutic dosage of curcumin for the prevention of radiation-induced lung injury in human will be more investigated. Second, although numerous lines of evidence suggest that curcumin is a potent anti-inflammatory and anti-fibrotic agent, the precise pharmacological mechanism is not clear. In the present study, we propose more direct anti-inflammatory and anti-fibrotic effects of curcumin by showing that it inhibits TNF-α, TNFR1, NF-κB, TGF-β1, collagen accumulation in lungs during two responses of both pneumonitis and fibrosis period 8 wks after radiation exposure. Furthermore, our study was not limited to the intracellular level, but also examined tissues changes such as the accumulation of macrophages and thickening of the alveolar septa. A comparison of tissue changes through histological grading is an important aspect of our study that differentiates it from the previous study.
In conclusion, the present study showed that long-term curcumin administration inhibits the radiation-induced inflammatory processes, including macrophage infiltration and pro-inflammatory cytokine expression, and attenuated fibrotic changes, including alveolar septal thickening and perivascular fibrosis. Thus, curcumin treatment might be a beneficial radioprotective agent for patients with radiation-induced pneumonitis.
ACKNOWLEDGEMENTS
We thank Eun Ae Jeong and Hyun Joo Shin for exellent technical assistance and Se In Hah (Cyrus Hah) for helpful English editing. This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science, and Technology (no. 2012-0000301).
References
1. Rothwell RI, Kelly SA, Joslin CA. Radiation pneumonitis in patients treated for breast cancer. Radiother Oncol. 1985; 4:9–14. PMID: 3929337.

2. Tarbell NJ, Thompson L, Mauch P. Thoracic irradiation in Hodgkin's disease: disease control and long-term complications. Int J Radiat Oncol Biol Phys. 1990; 18:275–281. PMID: 2105920.

3. Maasilta P, Holsti LR, Blomqvist P, Kivisaari L, Mattson K. N-acetylcysteine in combination with radiotherapy in the treatment of non-small cell lung cancer: a feasibility study. Radiother Oncol. 1992; 25:192–195. PMID: 1335155.

4. Thresiamma KC, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J Exp Biol. 1996; 34:845–847. PMID: 9014516.
5. Redlich CA, Rockwell S, Chung JS, Sikora AG, Kelley M, Mayne ST. Vitamin A inhibits radiation-induced pneumonitis in rats. J Nutr. 1998; 128:1661–1664. PMID: 9772133.

6. Antonadou D, Petridis A, Synodinou M, Throuvalas N, Bolanos N, Veslemes M, Sagriotis A. Amifostine reduces radiochemotherapy-induced toxicities in patients with locally advanced non-small cell lung cancer. Semin Oncol. 2003; 30(6 Suppl 18):2–9. PMID: 14727235.

7. Anscher MS, Kong FM, Jirtle RL. The relevanceof transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer. 1998; 19:109–120. PMID: 9567247.
8. Anscher MS, Chen L, Rabbani Z, Kang S, Larrier N, Huang H, Samulski TV, Dewhirst MW, Brizel DM, Folz RJ, Vujaskovic Z. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys. 2005; 62:255–259. PMID: 15850930.

9. O'Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol. 2003; 13:274–289. PMID: 12903016.
10. Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006; 65:876–881. PMID: 16751069.
11. Nakao S, Ogtata Y, Shimizu E, Yamazaki M, Furuyama S, Sugiya H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol Cell Biochem. 2002; 238:11–18. PMID: 12349897.
12. Hallahan DE, Virudachalam S, Kuchibhotla J. Nuclearfactor kappaB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endothelium. Cancer Res. 1998; 58:5484–5488. PMID: 9850083.
13. Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991; 57:1–7. PMID: 2062949.
14. Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999; 39:41–47. PMID: 10051376.

15. Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995; 270:24995–25000. PMID: 7559628.
16. Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V, Alarcón de la Lastra C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007; 7:333–342. PMID: 17276891.

17. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcuminleads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007; 73:1434–1445. PMID: 17291458.
18. Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li ST, Sun CH. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomed Environ Sci. 2009; 22:32–39. PMID: 19462685.

19. Thresiamma KC, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced genotoxicity. J Exp Clin Cancer Res. 1998; 17:431–434. PMID: 10089063.
20. Bhatia AL, Sharma A, Patni S, Sharma AL. Prophylactic effect of flaxseed oil against radiation-induced hepatotoxicity in mice. Phytother Res. 2007; 21:852–859. PMID: 17486687.

21. Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009; 8:47–53. PMID: 18981722.

22. Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, Solomides CC, Javvadi P, Koumenis C, Cengel KA, Christofidou-Solomidou M. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010; 173:590–601. PMID: 20426658.

23. Venkatesan N, Punithavathi D, Babu M. Protection from acute and chronic lung diseases by curcumin. Adv Exp Med Biol. 2007; 595:379–405. PMID: 17569221.

24. Rezvani M, Ross GA. Modification of radiation-induced acute oral mucositis in the rat. Int J Radiat Biol. 2004; 80:177–182. PMID: 15164799.

25. Jagetia GC, Rajanikant GK. Effect of curcumin on radiation-impaired healing of excisional wounds in mice. J Wound Care. 2004; 13:107–109. PMID: 15045805.

26. Lee KH, Rhee KH. Radioprotective effect of cyclo(L-phenylalanyl-L-prolyl) on irradiated rat lung. J Microbiol Biotechnol. 2008; 18:369–376. PMID: 18309286.
27. Müller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993; 187:233–256. PMID: 8330898.

28. Vergara JA, Raymond U, Thet LA. Changes in lung morphology and cell number in radiation pneumonitis and fibrosis: a quantitative ultrastructural study. Int J Radiat Oncol Biol Phys. 1987; 13:723–732. PMID: 3570895.
29. Guerry-Force ML, Perkett EA, Brigham KL, Meyrick B. Early structural changes in sheep lung following thoracic irradiation. Radiat Res. 1988; 114:138–153. PMID: 3353501.

30. Nicholas D, Down JD. The assessment of early and late radiation injury to the mouse lung using X-ray computerised tomography. Radiother Oncol. 1985; 4:253–263. PMID: 4081113.

31. Willner J, Vordermark D, Schmidt M, Gassel A, Flentje M, Wirtz H. Secretory activity and cell cycle alteration of alveolar type II cells in the early and late phase after irradiation. Int J Radiat Oncol Biol Phys. 2003; 55:617–625. PMID: 12573748.

32. Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environ Health Perspect. 1986; 70:261–291. PMID: 3549278.

33. Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995; 31:361–369. PMID: 7836090.

34. Nishioka A, Ogawa Y, Mima T, Jin YJ, Sonobe H, Kariya S, Kubota K, Yoshida S, Ueno H. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys. 2004; 58:1235–1241. PMID: 15001268.
35. Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000; 10:296–307. PMID: 11040330.

36. Kuroki M, Noguchi Y, Shimono M, Tomono K, Tashiro T, Obata Y, Nakayama E, Kohno S. Repression of bleomycin-induced pneumopathy by TNF. J Immunol. 2003; 170:567–574. PMID: 12496444.

37. Martinet Y, Rom WN, Grotendorst GR, Martin GR, Crystal RG. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987; 317:202–209. PMID: 3600711.

38. Vignaud JM, Allam M, Martinet N, Pech M, Plenat F, Martinet Y. Presence of platelet-derived growth factor in normal and fibrotic lung is specifically associated with interstitial macrophages, while both interstitial macrophages and alveolar epithelial cells express the c-sis proto-oncogene. Am J Respir Cell Mol Biol. 1991; 5:531–538. PMID: 1958380.
39. Büttner C, Skupin A, Reimann T, Rieber EP, Unteregger G, Geyer P, Frank KH. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997; 17:315–325. PMID: 9308918.

40. Yi ES, Bedoya A, Lee H, Chin E, Saunders W, Kim SJ, Danielpour D, Remick DG, Yin S, Ulich TR. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996; 20:339–352. PMID: 8872498.

41. Park KJ, Oh YT, Kil WJ, Park W, Kang SH, Chun M. Bronchoalveolar lavage findings of radiation induced lung damage in rats. J Radiat Res. 2009; 50:177–182. PMID: 19377267.

42. Xia DH, Xi L, Xv C, Mao WD, Shen WS, Shu ZQ, Yang HZ, Dai M. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor beta1 and tumor necrosis factor alpha. Med Oncol. 2010; 27:697–701. PMID: 19636975.
43. Sandur SK, Deorukhkar A, Pandey MK, Pabón AM, Shentu S, Guha S, Aggarwal BB, Krishnan S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009; 75:534–542. PMID: 19735878.
44. Charbeneau RP, Peters-Golden M. Eicosanoids: mediators and therapeutic targets in fibrotic lung disease. Clin Sci (Lond). 2005; 108:479–491. PMID: 15896193.

45. Yang HJ, Youn H, Seong KM, Yun YJ, Kim W, Kim YH, Lee JY, Kim CS, Jin YW, Youn B. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem Pharmacol. 2011; 82:524–534. PMID: 21669192.

46. Card JW, Voltz JW, Carey MA, Bradbury JA, Degraff LM, Lih FB, Bonner JC, Morgan DL, Flake GP, Zeldin DC. Cyclooxygenase-2deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am J Respir Cell Mol Biol. 2007; 37:300–308. PMID: 17496151.
Fig. 1
Effects of curcumin on alveolar inflammation in radiation-induced rat lung. (A) Representative photomicrographs of H & E-stained lung sections from control (CTL), radiation (RT), radiation+curcumin (RT+Cur), and curcumin (Cur) rats. Scale bar=200 µm. (B) Representative photomicrographs of immunostained F4/80 in rat lungs from each group. Scale bar=100 µm. Arrows indicate macrophage.
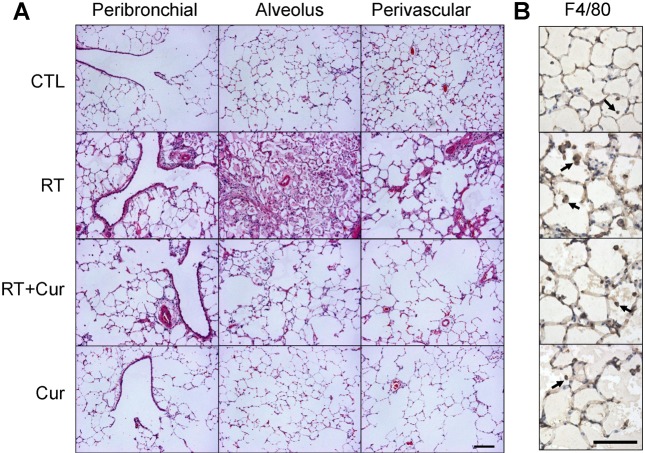
Fig. 2
Effects of curcumin on alveolar septal thickening and fibrosis in radiation-induced rat lungs. (A) Representative photomicrographs of H & E-stained lung sections from control (CTL), radiation (RT), radiation+curcumin (RT+Cur), and curcumin (Cur) rats. Scale bar=100 µm. (B) Alveolar septal thickness is presented as mean±SEM. (C) Representative photomicrographs of Masson's trichrome-stained lung sections from each group. Scale bar=200 µm. (D) Collagen content measured by Sircol assay in rat lungs (n=5~6 per group) after radiation. p<0.05 by ANOVA. *p<0.05, †p<0.001 vs. CTL and RT, respectively.
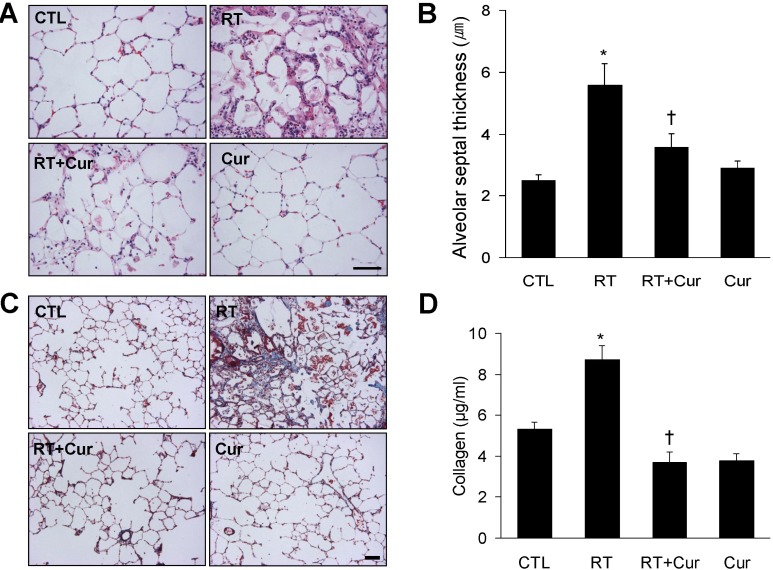
Fig. 3
Effects of curcumin on serum TGF-β1 and lung TGF-β1 and CTGF expression in irradiated rats. (A) Serum concentrations (n=5~6 per group) of TGF-β1 in control (CTL), radiation (RT), radiation+curcumin (RT+Cur), and curcumin (Cur) rats. (B) Western blot showing TGF-β1 and CTGF in lung from each group. (C) Quantification of lung TGF-β1 from western blot analysis. Densitometry values of each protein were normalized to α-tubulin and represented as arbitrary units (A.U.) relative to CTL levels. Data (n=5~6 per group) are presented as mean±SEM. *p<0.05 vs. CTL rats; †p<0.05 vs. RT rats.
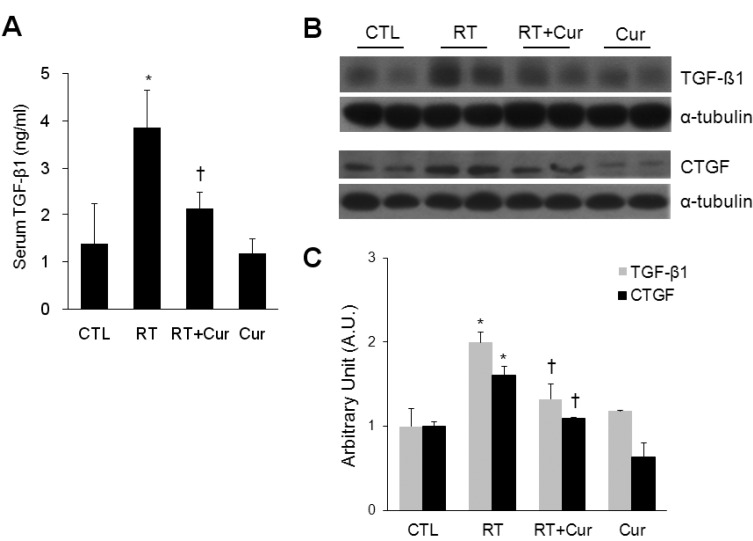
Fig. 4
Effects of curcumin on TNF-α, TNFR1, and TNFR2 expression in irradiated rat lung. (A) Western blot showing TNF-α, TNFR1, and TNFR2 in lungs from control (CTL), radiation (RT), radiation+curcumin (RT+Cur), and curcumin (Cur) rats. (B) Western blot quantification of lung TNF-α and TNFR1. TNFR2 expression was unchanged in all groups. Densitometry values of each protein were normalized to α-tubulin and represented as arbitrary units (A.U.) relative to CTL expression levels. Data (n=5~6 per group) are presented as mean±SEM. *p<0.05 vs. CTL rats; †p<0.05 vs. RT rats.
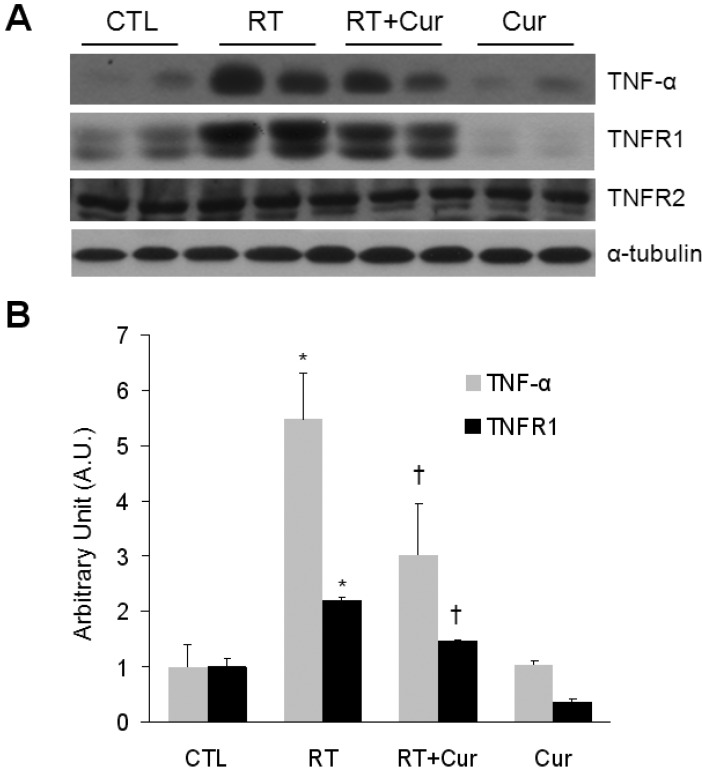
Fig. 5
Effects of curcumin on NF-κB p65 nuclear translocation in irradiated rat lung. (A) Western blot showing NF-κB p65 in the cytoplasm and nucleus of lungs from control (CTL), radiation (RT), radiation+curcumin (RT+Cur), and curcumin (Cur) rats. Cytosolic (cyto) and nuclear (nu) protein levels were normalized to β-actin and lamin A, respectively. (B) Representative photomicrographs of immunostained NF-κB p65 in lung from each group. Scale bar=100 µm. Arrows indicate macrophage. (C) Western blot showing acetylated NF-κB p65 in lungs from each group. Densitometry values for acetylated NF-κB p65 were normalized to α-tubulin and expressed as arbitrary units. Data (n=5~6 per group) are presented as mean±SEM. *p<0.05 vs. CTL rats; †p<0.05 vs. RT rats.
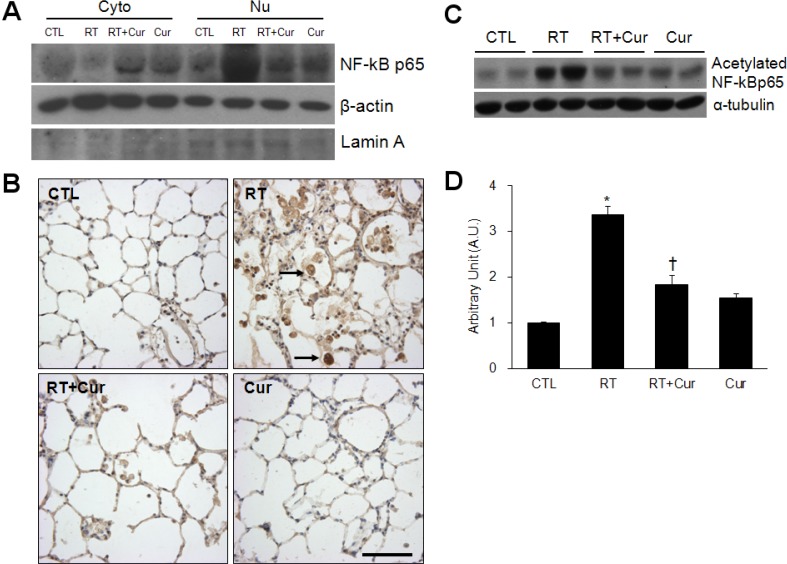
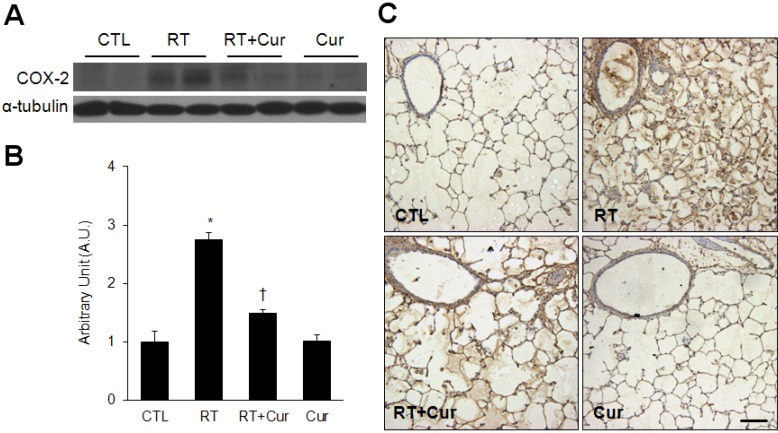
Fig. 6
Effects of curcumin on COX-2 expression in irradiated rat lung. (A) Western blot showing COX-2 in lungs from control (CTL), radiation (RT), radiation+curcumin (RT+Cur), and curcumin (Cur) rats. (B) Western blot quantification of lung COX-2. Densitometry values of each protein were normalized to α-tubulin and represented as arbitrary units relative to CTL. Data (n=5~6 per group) are presented as mean±SEM. *p<0.05 vs. CTL rats; †p<0.05 vs. RT rats. (C) Representative micrographs of immunostained COX-2 in lungs from each group. Scale bar=100 µm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download