Abstract
Allergic asthma is one of the most enduring diseases of the airway. The T-helper cells and regulatory T-cells are critically involved in inflammatory responses, mucus hypersecretion, airway remodelling and in airway hyper-responsiveness. Cigarette smoke (CS) has been found to aggravate inflammatory responses in asthma. Though currently employed drugs are effective, associated side effects demand identification and development of novel drugs with negligible or no adverse effects. Rutin, plant-derived flavonoid has been found to possess antioxidant and anti-inflammatory effects. We investigated the ability of rutin to modulate T-cells and inhibit inflammation in experimentally-induced asthma in cigarette smoke exposed mice. Separate groups of neonatal mice were exposed to CS for 10 days from post-natal days 2 to 11. After 2 weeks, the mice were sensitized and challenged with ovalbumin (OVA). Treatment group were given rutin (37.5 or 75 mg/kg body weight) during OVA sensitization and challenge. Rutin treatment was found to significantly inhibit cellular infiltration in the airways and Th2 and Th17 cytokine levels as well. Flow cytometry revealed effectively raised CD4+CD25+Fox3+ Treg cells and supressed Th17 cell population on rutin treatment. Airway hyper-responsiveness observed following CS and OVA challenge were inhibited by rutin. NF-κB and iNOS, chief regulators of inflammatory responses robustly activated by CS and OVA were down-regulated by rutin. Rutin also inhibited the expression of matrix metalloproteinase 9, thereby aiding in prevention of airway remodelling in asthma thereby revealing to be a potent candidate in asthma therapy.
Allergic asthma is a chronic airway disorder presenting with high rates of morbidity and mortality. Asthma is characterized by airway infiltration of inflammatory cells, airway remodelling, mucus hypersecretion and airway hyper responsiveness [1]. In asthma, allergen-induced inflammatory responses are chiefly mediated by T-helper type 2 (Th2) cells along with eosinophils as well mast cells and B cells [2]. Th2 cells synthesize high levels of interleukins (ILs) - IL-4, IL-5 and IL-13 [3] that activate the eosinophils and induce the production of allergen-specific immunoglobulin E (IgE) by B cells [4]. Interestingly, Th1 cells supresses Th2-mediated responses by secreting interferon IFN-γ. Th2 cytokine-IL-4 induces class switching in IgG1 and IgE, while IFN-γ is associated with IgG2α class switching. Thus Th1/Th2 cytokine balance is crucial in asthma [5].
Th17 cells, subsets of T lymphocyte, are reported to be involved in the pathogenesis of many immune disorders [6]. Th17 cells produce various cytokines as IL-17, IL-6, IL-22 and Tumor necrosis factor-α (TNF-α). IL-17 is found to induce the recruitment of airway macrophages [7]. Further, anti-IL-17 has been shown to reduce eosinophil infiltration in asthmatic model [8]. Another distinct T-cell subset, the regulatory T cells (Treg cells), have been found to be associated with asthma pathogenesis [9]. Treg cells expressing the forkhead/winged helix transcription factor (Foxp3) exert anti-inflammatory roles and also critically regulate Th2-induced allergic responses [10]. The regulatory T cells effectively maintain immune tolerance to self-components either by direct cell contact or by releasing IL-10, an anti-inflammatory cytokine [11].
NF-κB, a major transcription factor is found to play a pivotal role in the synthesis of Th2 cytokines and as well in the recruitment of inflammatory cells in the airways [12]. Accumulating studies have demonstrated the involvement of NF-κB in the pathogenesis of asthma [13]. Further, nitric oxide (NO) also exerts critical role in regulating airway function and is implicated in airway inflammation [14]. Numerous studies have reported down-regulation of inducible nitric oxide synthase (iNOS) inhibiting airway inflammatory responses in experimental models of asthma [15].
Matrix metalloproteinases (MMPs) play a key role in tissue modelling as seen in asthma and other airway inflammatory disorders [16]. MMP-9 is reported to be more involved in asthma and is found in serum, bronchoalveolar lavage fluid (BALF), sputum and transbronchial biopsy specimens of individuals with asthma [17]. Thus, MMP-9 is a potential molecular target in therapy against asthma.
Corticosteroids are used as the main drug for therapy of asthma [18]. However the inefficacy of the currently used drugs and also the associated local and systemic adverse effects on prolonged use [19] have forced the need for novel effective drugs [20] with negligible or no side effects. Much attention has been focussed on the use of plant-derived compounds as alternative therapy [21]. Rutin, a flavonoid found in citrus fruits such as grapefruits, lemon and oranges exhibits potent antioxidant properties [22] and also anti-inflammatory activities [23]. Cigarette smoke has been reported to trigger acute symptoms in asthma and the exposure of which has been found to strongly correlate with asthma severity [24]. The present study focussed on investigating the effects of rutin on cigarette smoke exposed asthmatic mice.
Antibodies against iNOS, p-NF-κBp65, p-Iκ-Bα, TNF-α, β-actin and MMP-9 used for expression studies were procured from Cell Signaling Technology (Beverly, MA, USA). Rutin and ovalbumin (OVA) were obtained from Sigma-Aldrich (St. Loius MO, USA). FITC-labelled anti-rat CD4, APC-labelled anti-rat CD25, phycoerythrin (PE) anti-mice IL-17A and PE-labelled anti-rat Foxp3 were procured from eBioscience Co. (San Diego, CA, USA). Wright-Giemsa stain was purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Unless stated, the other reagents and chemicals that were used in this study were procured from Sigma-Aldrich.
Female pregnant BALB/c mice were procured from animal centre of Shandong University. All the animals were housed in a sterile room (24±1℃ at 50~60% humidity, 12 h/12 h light/dark cycle) and were provided with water and food ad libitum. The mice were observed carefully for the birth of pups and the day of birth was noted as post-natal day 0. All the procedures were performed in compliance with the guide for the care and use of laboratory animals.
P2 mice (n=12/group) were used for the study. The mice were exposed to cigarette smoke (6 h/day) for 10 consecutive days from post-natal days 2 to 11 [2526]. The period from post-natal days 2 to 11 was chosen as it corresponds to the neonatal period in humans [27]. Exposure to cigarette smoke was carried out as described previously [26]. In brief the mice were randomly placed in an exposure chamber, measuring 1.92×1.92×0.97 m (3.58 m3) (BioClean, DuoFlo, Model H 5500, Lab Products Inc). Cigarette smoke from commercially available cigarettes was introduced into the exposure chamber at a rate of 4 cigarettes/15 min for 6 h/day by using a smoking machine (RM 1/G, Heinr Borgwald GmbH, Hamburg, Germany). The animals were exposed to sidestream smoke (passive smoke). At the end of the 6 h exposure period, an exhaust fan was turned on to clean the cigarette smoke from the chamber. Control animals were exposed to filtered air instead to cigarette smoke under identical conditions of temperature, humidity and flow rate. After every experimental exposure, the animals were transferred to respective cages. Two weeks later, the mice were sensitized and challenged with OVA as described previously by Oh et al. [28] with minor changes.
OVA (500 µg/ml in PBS) was mixed with equal volumes of 10% (w/v) aqueous aluminium potassium sulphate (alum) and incubated for 1 h at room temperature at pH 6.5. Following incubation, the mixture was centrifuged (750 g) for 5 min and the OVA/alum pellet was suspended in distilled water at its original volume. On the first day of sensitisation, the mice were administered with 0.2 ml of OVA (500 µg/ml solution in normal saline) intraperitoneally. Mice were then given 100 µl of OVA (2.5 mg/ml solution; 250 µg) on 8th day. On days 15, 18, and 21, the mice were challenged intranasally (i.n.) with OVA at 125 µg (50 µl of 2.5 mg/ml solution) as described by Oh et al. [28]. The treatment group mice were administered with rutin at 37.5 or 75 mg/kg b.wt orally, every day from day 1 of OVA sensitisation to day 21. On the days that the mice received OVA challenge rutin was administered 60 min prior before injections. Separate group of mice that received dexamethasone (Dex; 2 mg/kg; i.p) an hour before OVA injections served as positive control. The normal control mice received no OVA injections or rutin or were exposed to cigarette smoke. Mice that received CS and were challenged with OVA, but not treated with rutin were referred as asthmatic control.
After 24 h from the last OVA challenge, the mice were sacrificed by pentobarbital overdose (50 mg/kg) and tracheotomy was performed. Ice-cold PBS solution (0.5 ml) was infused into a lung and BALF was collected by three successive aspirations (total volume 1.5 ml) via tracheal cannulation [29]. The collected BALF was then centrifuged at 1,500 rpm for 10 min at 4℃. The supernatants were collected and stored at −80℃ for analysis of cytokine concentrations. The cell pellets were resuspended in PBS and the differential cell counts were determined by staining with Wright-Giemsa stain.
BALF levels of IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, and IFN-γ were determined by ELISA according to manufacturer's instructions. The kits for analysis of were purchased from R&D Systems (Minneapolis, MN, USA).
Whole blood from the mice of different groups was collected and the serum was separated and stored at −80℃. OVA-specific IgE levels in serum and in BALF were determined as described by Jain et al. [30]. Briefly, a 96-well microtitre plate coated with OVA (10 mg/ml) was treated with BALF or mouse sera followed by biotin-conjugated rat anti-mouse IgE (Pharmingen, San Diego, CA). Avidin horseradish peroxidase (HRP) solution was then added to each well and the absorbance was read at 405 nm.
The spleen was removed from each sacrificed mouse and placed in a tube containing RPMI1640 media (Gibco). A single cell suspension was prepared by disrupting the spleen using Cell disrupter (Beckman) and the cells were equally distributed into tubes and washed. For Treg analysis, cells were incubated with fluorescein isothiocyanate (FITC)-labelled anti-CD4 and APC-labelled anti-CD25 antibodies in staining buffer and incubated for 30 min at 4℃. Following surface staining, the cells were fixed, permeabilized and subsequently stained with PE coated-anti-rat Foxp3 and finally resuspended in staining buffer. Th17 analysis cells were incubated with FITC-labelled antihuman CD4 (4℃ for 30 min). Following surface staining, cells were fixed, permeabilized and stained with PE coated-anti-mice IL-17A and subjected to analysis by flow cytometry (FACS, Calibur instrument with CellQuest software; BD Biosciences, Mountain View, CA, USA).
AHR was determined as a measure of airway function following aerosolized methacholine challenge using Buxco's modular and invasive system (Buxco Electronics Inc., NY, USA). Changes in airway resistance (RI) and lung compliance (Cdyn) in response to various concentrations of methacholine were recorded directly as previously described by Pichavant et al. [31]. Briefly, the mice were anesthetized, tracheostomized and cannulated and laid inside a body plethysmograph chamber connected to the ventilator. The mice were observed for a stable baseline airway pressure (<5% variation over 2.5 min), and when achieved, the animals were administered aerosolized PBS or increasing concentrations of methacholine (3.125, 6.25, 12.5, or 25 mg/ml) via a jet nebulizer. RI and Cdyn values are expressed as percentage with respect to corresponding basal values recorded in response to PBS [32].
The lung tissues were collected and fixed in 10% formalin, paraffinized and 5 mm sections were cut and stained with hematoxylin and eosin (H&E). For examination of mucus production, periodic acid-fluorescence Schiff stain (PAFS) was employed. The mucin granules emit red fluorescence at excitation wavelength of 380~580 nm and were observed at 600~650 nm using Leica TCS SP5, confocal microscope (Leica Microsystems, IL, USA) as described by Bao et al. [33]. To assess the severity of leucocyte infiltration, a peribronchial cell count was done as previously described by Duan et al. [34]. The counts were based on a five point scoring system as - 0- no cells; 1- a few cells; 2- a ring of cells 1 cell layer deep; 3- a ring of cells 2–4 cell layers deep; and 4-a ring of cells more than 4 cell layers deep.
The lung tissues were washed with PBS and were homogenized, and incubated in lysis buffer with protease inhibitors (0.5 M EDTA, 5 M NaCl, 10% Nonidet P-40, 0.1 M phenylmethylsulfonyl fluoride, 0.1 M EGTA, 1 M sodium fluoride, 1 M HEPES, 0.2 M sodium orthovanadate, 2 µg/ml aprotinin, and 2 µg/ml leupeptin) to obtain extracts of lung proteins.
The lung tissue protein concentrations were determined by using protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The isolated proteins of each group were electrophoreitically separated using SDS PAGE (12%). The separated proteins were blotted and transferred on to nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk and incubated with primary antibodies (against MMP-9, iNOS, p-NF-κB p65 (ser 536), p-IκBα, TNF-α) overnight at 4℃ following incubation with peroxidase-conjugated secondary antibodies at room temperature for 1 h. The density of the immunoreactive bands was visualized with a chemiluminescene system (Amersham Bioscience, Buchinghamshire, UK). The band intensities were normalized to those of β-actin using anti-β-actin antibody.
The observed results are presented as mean±standard deviation (SD), taken from 6 independent experiments. The data were subjected to statistical analysis using SSPS package version 22.0. Values at p<0.05 obtained by one-way Analysis of variance (ANOVA) at p<0.05 followed by Duncan's Multiple Range Test (DMRT) for post hoc analysis are considered significant.
To investigate the effect of rutin (37.5 mg and 75 mg) on AHR in response to increasing concentrations of methacholine, we measured both RI and Cdyn in mechanically ventilated mice. Asthma is characterized by AHR, which is presented with excessive narrowing of the airways in response to a variety of allergic stimulus. RI is the pressure driving respiration divided by the air flow. Cdyn is lung distensibility and is defined as the change in lung volume due to change in pressure across the lung. OVA challenge developed airway hyper responsiveness that is typically reflected by high RI values and low Cdyn values (Fig. 1 and 2). The effects were more pronounced in mice that were subjected to neonatal cigarette smoke exposure followed by OVA challenge as compared against control. Rutin at the tested doses dramatically (p<0.05) reduced RI and restored Cdyn in response to methacholine. Rutin administration following CS exposure and during OVA challenge proved to be very effective in improving Cdyn. Further, the effects of rutin were found to be dose-dependent, with 75 mg dose exhibiting more positive results and the effects were comparable to dexamethasone.
Inflammation is considered as a hallmark in allergic asthma. We assessed the influence of rutin on the inflammatory cell infiltration in the BALF following OVA-induction. We examined the total cell counts in the BALF to determine the effects of rutin. Mice exposed to CS and then subjected to OVA challenge was found to exhibit severe eosinophilia and leukocyte infiltration in the BALF (Fig. 3). These counts were higher as compared to levels in mice that were not exposed to CS but were OVA-induced. However, rutin caused marked (p<0.05) suppression in eosinophil counts and also reduced lymphocytes, macrophages and neutrophils in the BALF. The 75 mg dose displayed higher suppressive effects in line with dexamethasone that was used as positive drug control.
Allergen-specific IgE on the surfaces of mast cells following an allergen challenge is evidenced as early events in asthma. The serum and BALF levels of OVA-specific IgE were determined 24 h after the final OVA challenge. We noticed drastic increase (p<0.05) in serum and BALF levels of OVA-specific IgE on CS exposure followed by sensitization and challenge with OVA. Rutin treatment of OVA-sensitized mice resulted in significant (p<0.05) reductions in OVA-specific IgE levels both in serum and in BALF (Fig. 4). Dexamethasone also caused supressed IgE levels.
To determine the effects of rutin on secretion of cytokine into BALF, IL-4, 5, 6, 10, 13, 17A and IFN-γ levels were measured. Th2 cytokines (IL-4, IL-5, and IL-13) and Th17 cytokines -IL-17A and IL-6 significantly increased (p<0.05) following CS and OVA challenge (Fig. 5 and 6), while the levels of Th1 cytokine - IFN-γ and IL-10, produced by Tregs were found to be reduced. Levels of Th2 and Th17 were lesser in mice exposed to OVA-alone compared to levels in mice exposed to CS and OVA challenged. Further, decrease in the levels of Th1 and Th17 cytokines with increased IL-10 and IFN-γ was observed in mice treated with rutin at 37.5 and 75 mg. The levels of IL-10 and IFN-γ in mice treated with 75 mg rutin was much close to normal values as compared to lower dose of 37.5 mg. Dexamethasone treatment also reduced Th2 cytokine levels.
CD4+CD25+Foxp3+ Tregs have been well documented in asthma, and Th17 cells, are known to be involved in allergic responses. Thus, in this study we determined the relative proportion of Treg cells and Th17 cells. Our study showed increased Th17 and decreased CD4+CD25+Foxp3+ Treg cells following CS and OVA challenge (Fig. 7). The cell counts were much low in mice that were treated with rutin and exposed to OVA-alone and not exposed to CS compared to the counts in mice that were exposed to CS and challenged with OVA. Interestingly, administration of rutin enhanced Treg cells counts in a dose-dependent manner while decreasing Th17 cells compared to the OVA-challenged group. The 75 mg dose of rutin was found to be more effective than the 37.5 mg dose.
Histological studies were carried out to assess any changes in the architecture of the lung tissues. The results of the H&E analysis revealed marked infiltration of inflammatory cells into the peribronchiolar and perivascular connective tissues following OVA challenge, however, the infiltration was more severe in mice that were exposed to CS and OVA as compared to the mice that were not exposed to CS but challenged with OVA (Fig. 8A and B). We observed that treatment with rutin (37.5 or 75 mg/kg) supressed infiltration of eosinophils and neutrophils in the lung tissues. Rutin was also found to considerably restore normal lung histology. Rutin at 75 mg dose exhibited almost near normal histology with negligible cellular infiltration. Asthma is characterised with mucus hypersecretion and AHR. Here marked goblet cell hyperplasia with hypersecretion of mucus was observed in OVA challenged mice, that were or not exposed to CS, however CS was found to exacerbate mucus secretion. Reduction in goblet cell hyperplasia was seen in rutin treatment (Figs. 9A and B). While dexamethasone treatment presented tissue sections with almost no mucus hypersecretion alterations and with negligible cell infiltration.
Persistent activation of NF-κB has been observed in allergic airway inflammation. In our study cigarette smoke and OVA challenge resulted in a robust increase in the expression of p-NF-κB p65 (ser 536), p-IκBα and TNF-α (Figs. 10A~D). Rutin administration resulted in marked down regulation of p-NF-κB p65, p-IκBα and TNF-α. Neonatal exposure to CS was found to bring out more marked activation of NF-κB signaling than exposure to OVA alone. Further, elevated iNOS gene expression upon CS and OVA challenge were significantly (p<0.05) down-regulated by rutin.
Studies have demonstrated the involvement of MMP-9 in respiratory tract diseases as asthma that is associated with progressive remodelling of the airway wall [35]. In this study we noticed significantly (p<0.05) elevated MMP-9 expression in the lung tissues of animals that were challenged with OVA (Figs. 10A~D). These elevations were observed more in mice that were exposed to CS and then sensitized and challenged with OVA. The increased expression was found to correlate with altered histology of the lung tissues and alterations in the peribhonchioloar connective tissues. Rutin down-regulated MMP-9 expression with 75 mg dose exhibiting higher inhibition. However, dexamethasone was found not to have much effect on the expression of MMP-9. These observations suggest that rutin was able to also aid in restoring the architecture of airway and lung tissues following asthmatic challenge.
Asthma is one of the most common chronic lung diseases characterized by inflammation, reversible airway obstruction, hypersecretion of mucus and infiltration of inflammatory cells into the airways [36]. Cigarette smoke exposure is considered as a risk factor in many allergies and has been reported to promote allergic inflammation and also CS has been found to exacerbate allergic asthma [37]. We observed increased cellular infiltration in the BALF of the mice exposed to CS and/or OVA. However the inflammatory cell counts were found higher on exposure to CS+OVA as compared to OVA challenge alone.
Cigarette smoke has been found to increase the levels of inflammatory cells as macrophages, neutrophils and also goblet cells that cells play critical roles in the exacerbation of asthmatic airway inflammation [38]. It is well known that migration of inflammatory cells into the lung is a key contributor in allergic airway inflammation [39]. In asthma, bronchoconstriction due to inflammation and contraction or hypertrophy of airway smooth muscles eventually leads to decreased function of the lungs [40]. In our study, rutin was found to significantly reduce inflammatory cell counts and eosinophilia following CS and OVA challenge. Further, CS exposure caused an increase in AHR in comparison to mice exposed to OVA-alone. Rutin improved lung compliance and reduced resistance of the airway. The decreased cell infiltration on rutin administration could have possibly aided in reduced bronchoconstriction thereby improving lung function.
Th2 cytokines have been well documented to be critically involved in the pathogenesis of allergic airway inflammation [12]. Together with Th2 cytokines (IL-4, IL-5 and IL-13), Th17 cytokines (IL-17A, IL-6) also are known to play vital roles in asthma [41]. IFN-γ, secreted by the Th1 cells suppresses the immune response by Th2 cytokines [5]. The cytokines IL-4 and -13 stimulate IgE production, while IL-5 contributes to eosinophilia and cellular infiltration into the airways [42]. IL-17 is involved in neutrophil-dominated inflammation and promotes neutrophil accumulation and activation in the lung [43]. We noticed a drastic increase in the levels of ILs-4, 5, 6, 13 and 17A in the BALF following CS exposure and/or OVA challenge. Also, the increase was higher on exposure to both CS and OVA than in OVA challenge alone. Previous studies have shown that CS can induce secretion of inflammatory cytokines [38]. Thus, the findings suggest possible stimulation of cytokines by CS. Rutin reduced the aggravated Th2 and Th17 cytokine levels while it enhanced the production of IFN-γ. This stimulation also aids in reducing the intensity of inflammation. Rutin-mediated reduced Th2 cytokines and raised IFN-γ could possibly be responsible in reduction of OVA-specific IgE levels observed both in serum and BALF.
The observation of H&E staining of the lung tissue revealed decrease in inflammatory cytokine levels in line with the extent of cellular infiltration in the peribronchiolar area in rutin treatment. PAS staining indicated suppression of mucus hypersecretion by rutin. The altered lung histology was also found to be restored on rutin administration. Studies have shown that Th2 cytokines are involved in goblet cell hyperplasia, mucus hypersecretion and airway remodelling [44]. The suppression of IL-4, 5 and 13 by rutin could have contributed to the decrease in PAS positive scores indicating decreased mucus secretion. These observations suggest the potent anti-inflammatory activity of rutin by supressing Th2 and as well Th17 cytokines.
Further, flow cytometry was done to analyse the population of CD4+CD25+Fox3+ Tregs and Th17 cells. The CD4+CD25+Fox3+ Tregs secrete IL-10 that has anti-inflammatory effects. Royer et al. [45] reported that IL-10 could inhibit activation of mast cells. IL-10 was found to abolish AHR and also airway eosinophilia in experimental animal model of asthma [28]. Our data demonstrated that rutin can enhance CD4+CD25+Tregs, increase IL-10 level in BALF and as well decrease Th17 cell populations.
The expression of NF-κB and iNOS were assessed. NF-κB and iNOS mediated signaling are chief pathways involved in inflammatory responses leading to synthesis of cytokines and inflammatory mediators. NF-κB is a crucial transcription factor for Th2 cell differentiation [46] and activation of NF-κB is critical for airway remodelling and inflammation [47]. In addition, iNOS gene expression is tightly regulated by NF-κB signaling [48]. In our study, significantly up-regulated expression of p-NF-κBp65, p-IκBα, TNF-α and iNOS were observed on CS and OVA challenge. Suresh et al. [49] has reported that IL-13 induces iNOS expression in human bronchial epithelial cells leading to elevate NO production. Down-regulated expression of p-NF-κBp65 in rutin administrated mice suggests inhibition of the pathway. Rutin also caused decreased iNOS expression. However, this reduction could be either due to direct effects of rutin or in part due to suppression of NF-κB activation or due to suppression of IL-13.
Airway remodelling is a typical characteristic in asthma and MMP-9 is recognized as a key factor involved in asthma-associated structural changes of airway [50]. MMP-9 also modulates inflammatory responses by interacting with the cytokine/chemokine networks, including catalytic activation of IL-8 and release of IL-13 [51]. Neovastat (AE-941), a natural MMP-9 antagonist, display has been shown to be of benefit in in murine model of asthma [35]. We observed dramatically up-regulated expression of MMP-9 on exposure to CS. The expression was higher on exposure to CS and OVA. Significant down-regulation of MMP-9 was observed on rutin administration thus exhibiting protective effects. MMP-9 suppression could also be responsible for the restoration of histology of lung tissue by rutin.
CS exposure exacerbated inflammatory responses in asthma. The flavonoid rutin was found to possess potent anti-inflammatory effects in both CS exposed and non-exposed animals. Rutin effectively inhibited inflammatory cytokines and improved lung function and as well inhibited AHR. Overall the results of the study revealed that rutin could be explored further as an antiasthmatic candidate drug.
Figures and Tables
 | Fig. 1Rutin reduced airway resistance in lung function following CS and OVA challenge.Data are given as mean±SD where n=6. *Symbolizes p<0.05 related with control as determined by one way-ANOVA.
|
 | Fig. 2Rutin improved lung compliance following CS and OVA challenge.Data are given as mean±SD where n=6. *Symbolizes p<0.05 related with control as determined by one way-ANOVA.
|
 | Fig. 3Effect of Rutin on cell accumulation in BALF.Rutin reduced inflammatory cell infiltration in to BALF following CS and OVA challenge. Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~gdenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis.
|
 | Fig. 4Rutin reduces OVA-specific IgE levels in serum and BALF.Rutin was found to significantly reduce the raised OVA-specific IgE in serum and in the BALF in mice exposed to CS and/or OVA. Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~idenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis.
|
 | Fig. 5Rutin caused marked decrease in the levels of Th2 cytokines and increased the levels of IFN-γ.Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~idenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis.
|
 | Fig. 6Rutin caused marked decrease in the levels of Th17 cytokines and increased the levels of IL-10.Data are given as mean±SD where n=6. adenotes statistical significance at p<0.05 related against control and b~idenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis.
|
 | Fig. 7Rutin regulates Th17 and CD4+CD25+Foxp3+ Treg cell populations.Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~hdenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis.
|
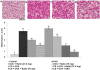 | Fig. 8Rutin was found to reduce inflammatory cell infiltration as in H&E staining (A) and reduce inflammation score (B).Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~hdenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis. (a) Control, (b) OVA+Rutin (75 mg), (c) CS+OVA+Rutin (75 mg), (d) CS+OVA+DEX.
|
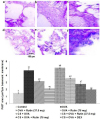 | Fig. 9Rutin significantly reduce mucus hypersecretion and goblet cell hyperplasia as in PAFS staining (A and B).Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~hdenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis. (a) Control, (b) OVA, (c) OVA+Rutin (75 mg), (d) CS+OVA, (e) CS+OVA+Rutin (75 mg), (f) CS+OVA+DEX.
|
 | Fig. 10Rutin modulates NF-κB/iNOS-mediated signaling and reduce MMP-9 expression.(A) L1, Control; L2, OVA; L3, OVA+Rutin (37.5 mg); L4, OVA+Rutin (75 mg); L5, CS+OVA; L6, CS+OVA+Rutin (37.5 mg); L7, CS+OVA+Rutin (75 mg); L8, CS+OVA+DEX. (B~D) Data are given as mean±SD where n=6. aDenotes statistical significance at p<0.05 related against control and b~hdenotes data within the same group that differ from each other at p<0.05 as calculated by one-way ANOVA followed by DMRT analysis.
|
ACKNOWLEDGEMENTS
This study was supported by the Foundation for Outstanding Young Scientist in Shandong Province (BS2011YY018).
Notes
References
1. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.

2. Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008; 26:205–232.

3. Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011; 12:114.

4. Nakajima H, Hirose K. Role of IL-23 and Th17 cells in air way inflammation in asthma. Immune Netw. 2010; 10:1–4.
5. Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999; 163:6448–6454.
6. Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007; 120:247–254.

7. Sergejeva S, Ivanov S, Lötvall J, Lindén A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005; 33:248–253.

8. Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003; 28:42–50.

9. Boulet LP, Turcott H, Plante S, Chakir J. Airway function, inflammation and regulatory T cell function in subjects in asthma remission. Can Respir J. 2012; 19:19–25.

11. Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006; 212:8–27.
12. Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004; 4:1817–1828.
13. Gagliardo R, Chanez P, Mathieu M, Bruno A, Costanzo G, Gougat C, Vachier I, Bousquet J, Bonsignore G, Vignola AM. Persistent activation of nuclear factor-kappaB signaling pathway in severe uncontrolled asthma. Am J Respir Crit Care Med. 2003; 168:1190–1198.
14. Jeon WY, Shin IS, Shin HK, Lee MY. Samsoeum water extract attenuates allergic airway inflammation via modulation of Th1/Th2 cytokines and decrease of iNOS expression in asthmatic mice. BMC Complement Altern Med. 2015; 15:47.

15. Yoo D, Guk K, Kim H, Khang G, Wu D, Lee D. Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int J Pharm. 2013; 450:87–94.

16. Elias JA. Airway remodeling in asthma. Unanswered questions. Am J Respir Crit Care Med. 2000; 161:S168–S171.
17. Bossé M, Chakir J, Rouabhia M, Boulet LP, Audette M, Laviolette M. Serum matrix metalloproteinase-9:Tissue inhibitor of metalloproteinase-1 ratio correlates with steroid responsiveness in moderate to severe asthma. Am J Respir Crit Care Med. 1999; 159:596–602.

18. Tritar-Cherif F, Ben M'Rad S, Merai S, Djenayah F. Corticotherapy for asthma in the child. Tunis Med. 2002; 80:1–6.
19. Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol. 2007; 275:98–108.

20. Li XM, Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J Allergy Clin Immunol. 2009; 123:297–306. quiz 307–308.

21. Cho SJ, Kim HW, Kim BY, Cho SI. Sam So Eum, a herb extract, as the remedy for allergen-induced asthma in mice. Pulm Pharmacol Ther. 2008; 21:578–583.

22. Metodiewa D, Kochman A, Karolczak S. Evidence for antiradical and antioxidant properties of four biologically active N,N-diethylaminoethyl ethers of flavanone oximes: a comparison with natural polyphenolic flavonoid (rutin) action. Biochem Mol Biol Int. 1997; 41:1067–1075.
23. Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001; 56:683–687.

24. Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Epidemiological study on the Genetics and Environment of Asthma. Eur Respir J. 2000; 15:470–477.
25. Barrett EG, Wilder JA, March TH, Espindola T, Bice DE. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Respir Crit Care Med. 2002; 165:1410–1418.

26. Wu ZX, Benders KB, Hunter DD, Dey RD. Early postnatal exposure of mice to side-steam tobacco smoke increases neuropeptide Y in lung. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L152–L159.

27. Pinkerton KE, Joad JP. The mammalian respiratory system and critical windows of exposure for children's health. Environ Health Perspect. 2000; 108:Suppl 3. 457–462.

28. Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, Moon SH, Cao B, Ogbu C, Jeong KW, Kozu G, Nakanishi H, Kahn M, Chi EY, Henderson WR Jr. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002; 168:1992–2000.

29. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990; 142:434–457.

30. Jain VV, Kitagaki K, Businga T, Hussain I, George C, O'shaughnessy P, Kline JN. CpG-oligodeoxynucleotides inhibit airway remodeling in a murine model of chronic asthma. J Allergy Clin Immunol. 2002; 110:867–872.

31. Pichavant M, Goya S, Hamelmann E, Gelfand EW, Umetsu DT. Animal models of airway sensitization. Curr Protoc Immunol. 2007; Chapter 15:Unit 15.18.

32. Glaab T, Daser A, Braun A, Neuhaus-Steinmetz U, Fabel H, Alarie Y, Renz H. Tidal midexpiratory flow as a measure of airway hyperresponsiveness in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2001; 280:L565–L573.

33. Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, Leung BP, Wong WS. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009; 179:657–665.
34. Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004; 172:7053–7059.

35. Lee YC, Lee HB, Rhee YK, Song CH. The involvement of matrix metalloproteinase-9 in airway inflammation of patients with acute asthma. Clin Exp Allergy. 2001; 31:1623–1630.

37. Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res. 2006; 7:49–57.

38. Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006; 291:L46–L57.
39. Elsner J, Kapp A. Regulation and modulation of eosinophil effector functions. Allergy. 1999; 54:15–26.

41. Li J, Zhang B. Apigenin protects ovalbumin-induced asthma through the regulation of Th17 cells. Fitoterapia. 2013; 91:298–304.

44. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006; 118:551–559. quiz 560–561.

45. Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy. 2001; 31:694–704.

46. Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009; 123:986–994. quiz 995–996.

47. Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005; 5:435–445.
48. Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS. IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: role of MAPK (Erk1/2, JNK, and p38) pathways. J Immunol. 2006; 177:4064–4071.

49. Suresh V, Mih JD, George SC. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007; 37:97–104.

50. Grzela K, Zagorska W, Krejner A, Litwiniuk M, Zawadzka-Krajewska A, Banaszkiewicz A, Kulus M, Grzela T. Prolonged treatment with inhaled corticosteroids does not normalize high activity of matrix metalloproteinase-9 in exhaled breath condensates of children with asthma. Arch Immunol Ther Exp (Warsz). 2015; 63:231–237.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download