Abstract
Cedrus deodara (Pinaceae) has been used traditionally in Ayurveda for the treatment of central nervous system disorders. 3,4-bis(3,4-dimethoxyphenyl)furan-2,5-dione (BDFD) was isolated from heart wood of Cedrus deodara and was shown to have antiepileptic and anxiolytic activity. Thus, the present study was aimed to explore its anti-depressant effect and to correlate the effect with serotonin and nor adrenaline levels of brain. Albino mice were used as experimental animal. Animals were divided in to three groups; vehicle control, imipramine (30 mg/kg i.p.), BDFD (100 mg/kg i.p.). Tail suspension test (TST) and forced swim test (FST) was performed to evaluate antidepressant effect of BDFD. BDFD (100 mg/kg, i.p.) showed a significant decrease in immobility time when subjected to FST whereas immobility time was not significantly altered in TST. BDFD treatment increased serotonin and noradrenaline levels in the brain which is indicative of BDFD having possible atypical antidepressant action.
Go to : 
Cedrus deodara is a tree that grows in high altitudes of Himalayan regions of eastern Afghanistan, northern Pakistan, northern India, and western Nepal. This tree is considered to be a divine tree in the Hindu mythology. Medicinal properties of this tree are well recorded in Ayurvedic text. The aromatic odour of cedrus oil believes to cure various ailments and is one of the popular ingredients in aromatherapy. Various extracts or constituents of this tree has been reported for a number of medicinal uses viz., spasmolytic [1], immuno-modulatory [2], anti-cancer [3], anti-fungal [4], disinfectant [5], anti-arthritic [6], anti-allergic [7], anti-oxidant [8], anti-filarial [9], and molluscicidal [10], antidiabetic [11], antitubercular [12], antiuro-lithiatic [13], antisecretory and antiulcer [14] activities.
The major phyto-constituents of heart wood of C. deodara are sesqui-terpenes and glycosides. Cedeodarin (6-methyltaxifolin), wikstromol, dihydromyricetin (ampelopsin), cedrin (6-methyldihydromyricetin), cedrinoside, deodarin, (-)-nortrachelogenin, (-)-matairesinol and a dibenzylbutyrolactollignan (4,4',9-trihydroxy-3,3'-dimethoxy-9,9'-epoxylignan) are some of the phytocompounds isolated from C. deodara wood. Recently, we had isolated anxiolytic and antiepileptic principles from heart wood of C. deodara and characterized it to be 3,4-bis(3,4-dimethoxyphenyl)furan-2,5-dione (BDFD). During those studies, we estimated the influence of BDFD on brain GABA [15]. It was observed that BDFD treatment increased the brain GABA [15] levels. Currently available anti-depressant drugs, act by increasing the serotonin or nor-adrenaline levels or both in specific regions of brain by inhibiting its reuptake mechanisms. Also, GABA hypothesis of depression suggest that decreased levels of brain GABA levels and alteration in GABAA receptors can lead to depression. Further C. deodara is also used as brain tonic in folklore medicine. Hence, we hypothesized BDFD may possess antidepressant activity by raising serotonin, noradrenaline and GABA levels of brain.
To verify this we designed experiments to screen BDFD (100 mg/kg) for antidepressant activity in mice using tail suspension and force swim methods and its effects on brain monoamines viz., serotonin and nor-adrenaline levels in brain.
Go to : 
Albino mice, weighing 20~25 g of either sex procured from Central Animal Research Facility (CARF) of Manipal University, Manipal were used. Wistar rats, weighing 150~180 g of either sex were procured from Farooqia College of Pharmacy, Mysore were used.
The experimental protocols were approved by the Institutional Animal Ethics Committee. Before the experiment, animals were acclimatized and maintained under controlled conditions of temperature (23±2℃), humidity (50±5%). The animals were kept under standard conditions of 12 h light/dark cycle in sanitized polypropylene cages containing sterile paddy husk as bedding with free access to food and water ad libitum. Experiments were carried out in accordance with the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The lighting, temperature and noise level in the test room were kept constant during the study.
The test substance BDFD was generously supplied by one of our co-authors (Mr. D. Dhayabaran), Farooqia College of Pharmacy, Mysore. Isolation, purification characterisation, anxiolytic and antiepileptic activity of this test substance were reported earlier and the selection of dose for the present study was based on the anxiolytic activity of BDFD [15].
Imipramine (Antidep®, Torrent Pharmaceutical Limited, India) was taken as the standard antidepressant drug. BDFD (100 mg/kg) and imipramine (30 mg/kg) were administered 60 minutes before the test after suspending them in 0.3% sodium carboxy methylcellulose (CMC) in distilled water through intra-peritoneal (i.p.) route at a volume of 0.1 ml/10 g body weight of mice. Animals were randomized and divided in to 3 groups (n=6). The groups were as follows:
Group 1-Vehicle control (0.3% CMC 5 ml/kg i.p.)
Group 2-Imipramine (30 mg/kg i.p.)
Group 3-BDFD (100 mg/kg i.p.)
Mice were suspended 58 cm above the floor, on the edge of the table with the help of adhesive tape placed approximately 1 cm from the tip of the tail. The three groups of mice were suspended and total duration of immobility was recorded for 6 minutes using a stopwatch.
Mice are individually forced to swim inside a vertical Plexiglass cylinder of 45 cm height, diameter of 20 cm, containing 30 cm of water maintained at 27℃ for 6 minutes. Animal is considered to be immobile, if it remains floating passively in water with its nose just above the surface. After 23 h, mice are dosed with vehicle or test substance or imipramine (30 mg/kg, i.p). One hour after drug treatment the mice were placed in the cylinder and the total duration of immobility was measured during a 6 min test.
Rats were divided into four groups of six rats each and treated with vehicle control (3% Tween 80, p.o.) and BDFD (10, 30 and 100 mg/kg, p.o.) for four days.
Standard stock solutions (1 mg/ml) were prepared in hydrochloric acid (0.1 M) and kept in storage at -20℃. Fresh working standard solutions were prepared for each experiment in 0.1 M perchloric acid. Injection volume was 100 µl of nor adrenaline (NA) and 5-hydroxytryptamine (5-HT) containing 3 ng each. Monoamine levels in the tissue were identified by comparing their retention time and quantified by comparing their peak heights of samples with standards in elution profile.
Forty five minutes after the treatment, rats were sacrificed and heads were dropped into ice-cold 0.1 M perchloric acid. Immediately after the sacrifice of rats, brains were removed and transferred to ice chilled petri-dish. The tissue (brain) was weighed and homogenized in 2 ml of 0.1 M perchloric acid containing isoproterenol (internal standard, 30 ng/ml). The homogenate was centrifuged at 14,000 rpm for 15 min at 4℃. The supernatant was filtered by using a 0.2 ml membrane filter and 100 µl of the filtrate was injected to a HPLC column (C18 RP-18 column 250×4.6 mm, 5 µm). 5-HT and NA were detected at the excitation and emission wavelength of 280 nm and 315 nm respectively. The slit width was kept at 10/10 for excitation/emission respectively.
Brain monoamine levels were measured by HPLC method. The mobile phase was consisting of sodium acetate (0.02 M), methanol (16%), heptane sulphonic acid (0.055%), EDTA (0.2 mM) and dibutylamine (0.01%, V/V). The solution was filtered through 0.45 µm membrane filter after adjusting pH to 3.92 with o-phosphoric acid and degassed on a sonicator. The flow rate was 0.9 ml/min [19].
All the data are expressed as mean±SEM. Results were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test using Graph Pad Prism version 5.0 software and p<0.05 was considered statistically significant.
Go to : 
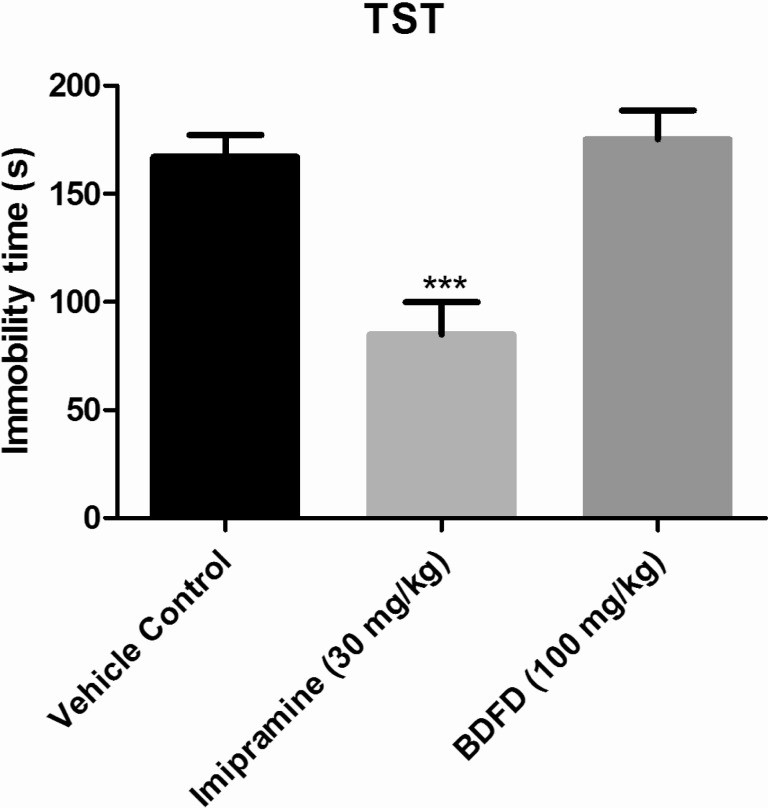
Immobility time in the imipramine treated group (84.83±15.09 sec) was significantly (p<0.001) decreased to almost half of the immobility time of the vehicle control group mice (167±10.22 sec). Treatment by BDFD fraction did not show any significant (p<0.05) change in immobility time (175.3±13.17) compared to vehicle control group (Fig. 1).
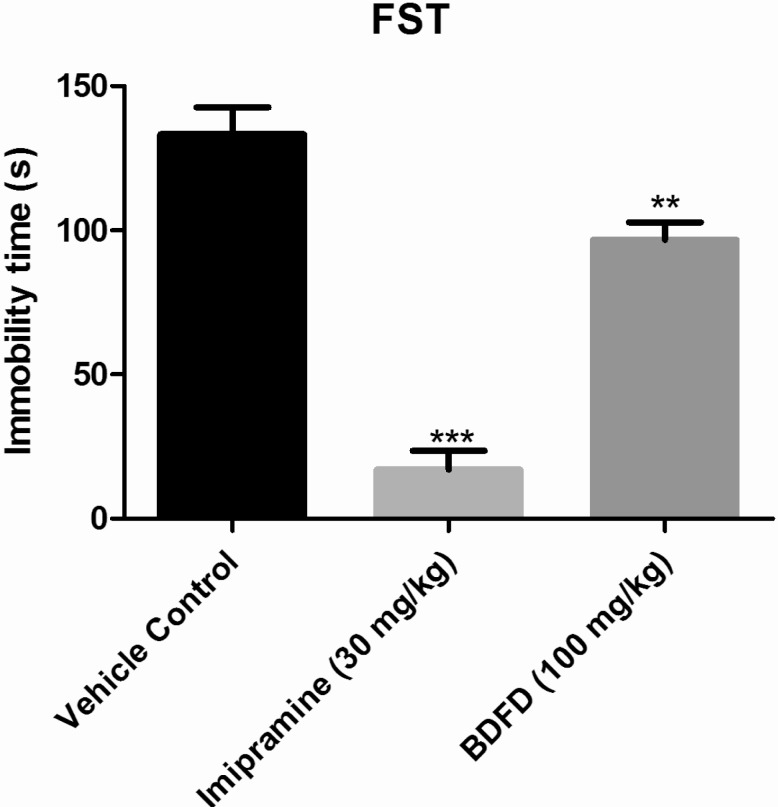
Immobility time in the imipramine treated group (17.00±6.62 sec) was decreased significantly (p<0.001) compared to vehicle control group (133.3±9.35 sec). Treatment of BDFD also found to be significantly (p<0.01) effective in decreasing immobility time (96.67±6.18 sec) compared to vehicle control group (Fig. 2).
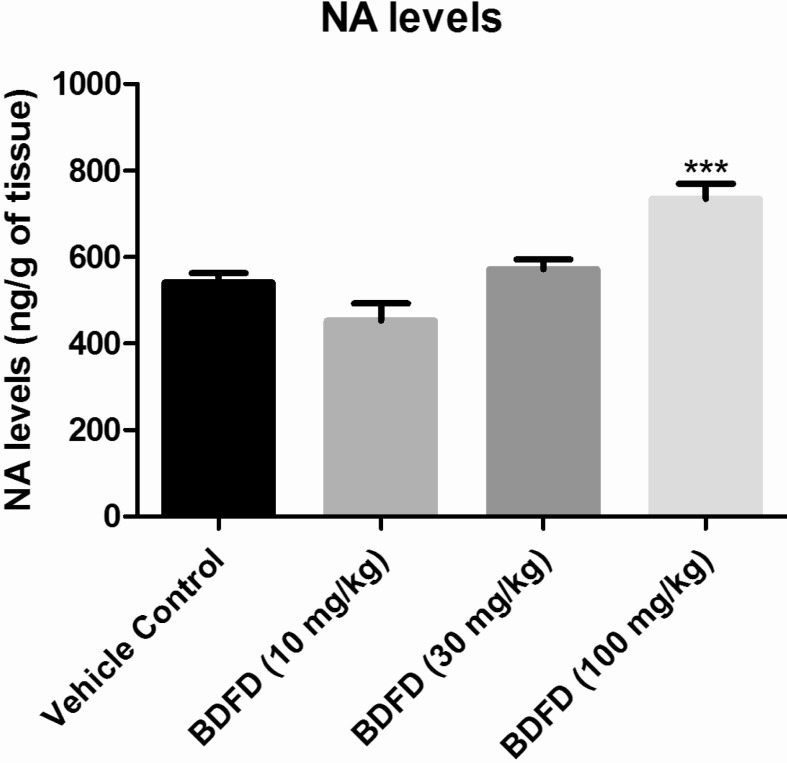
The brain NA level of vehicle control was found to be 541.13±21.51 ng/g of tissue. BDFD treatment showed a dose dependant increase in NA level in brain homogenate. The increase was found to be significant (p<0.001) compared to vehicle control (734.25±35.15 and 541.13±21.51 ng/g of tissue respectively) (Fig. 3).
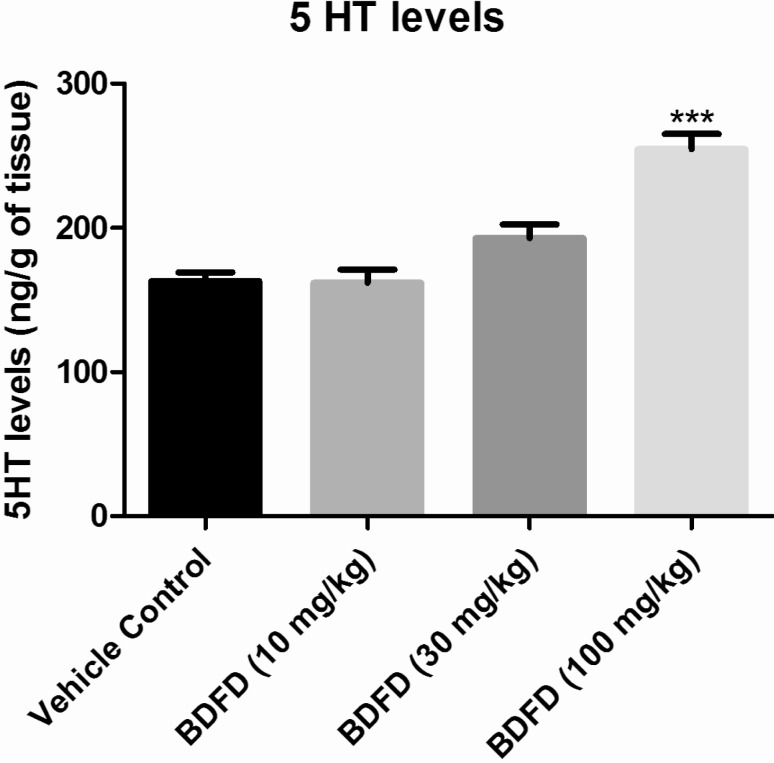
The serotonin levels in the brain of vehicle control were found to be 162.13±6.12 ng/g of tissue. BDFD treatment in rat showed a dose dependant increase in 5HT level. The increase was found to be significant (p<0.001) compared to vehicle control (254.63±10.41 and 162.13±6.12. respectively) (Fig. 4).
Go to : 
Mood is an emotional state, which varies with a number of factors. However, long-term disturbances of mood like depression and bipolar disorder are considered mood disorders or affective disorders [20]. The changes in the brain monoamine neurotransmitters specifically serotonin, dopamine and noradrenaline has been observed in patients presented with symptoms of major depression [21]. Apart from these, several neurotransmitters like GABA [22], glutamate [23] and acetylcholine [24] are also considered to be involved in the pathophysiology of depressive disorders. Various theories of depression have been proposed, linking these neurotransmitter levels to depression. Negative symptoms associated with depression are largely attributed to the decreased levels of serotonin, noradrenaline and GABA levels in various regions of brain. Most of the currently available antidepressant drugs exert its action through its interaction with monoamine neurotransmitters in the brain. In our previous study, we observed that BDFD treatment increased GABA [15] levels in the whole brain of rat. In the present study BDFD raised brain monoamine levels namely serotonin and nor-adrenaline. Therefore, we hypothesized that BDFD could reduce the negative symptoms associated with depression.
To verify the hypothesis, a set of experiments were designed to evaluate the antidepressant activity of BDFD in mice. The dose used was 100 mg/kg, since this dose produced maximum anxiolytic activity and significant increase in both serotonin and nor adrenaline levels in whole brain. Tail suspension test (TST) and force swim test (FST) are well validated and widely used tests to screen antidepressant activity in rodents [25,26,27]. When the mice are subjected to stress, a state of immobility is displayed by them, which may reflect the depressive behaviour in humans. Further, these tests produce a paradigm known as behavioural despair, which is closely associated with the negative signs of depression seen in humans [25,28]. Clinically effective antidepressants reduce the immobility time.
BDFD showed a significant decrease in immobility time of the rodents when subjected to FST whereas it did not significantly alter the immobility time in TST. Both TST and FST tests are likely to produce false positive results with certain drugs like psychomotor stimulants, which could decrease immobility time by stimulating locomotor activity [29]. However, in our previous experiments with BDFD (100 mg/kg), it did not improve the locomotor activity in rota-rod test, instead this drug produced motor in-coordination at 10 times the higher dose levels. Hence, the significant decrease in immobility seen in FST may not be associated with motor activity. Moreover, imipramine (30 mg/kg) significantly reduced the immobility time as compared to vehicle control in FST and TST indicating the validation of the experiment conducted in our laboratory.
It has been observed that some of the clinical atypical antipsychotic drugs used to treat depression in humans produced positive results in FST but failed to show its effect in TST in animal models [29]. The ability of BDFD to significantly reduce the behavioural despair in FST but not in TST may be attributed to its atypical antidepressant like effect in mice. Based on the evaluation of brain monoamine levels, we propose that BDFD may possess antidepressant activity similar to conventional antidepressant drugs; however, the results from TST and FST suggest that BDFD may have atypical antidepressant effect. Further studies are required to elucidate the mechanism of BDFD for its atypical antidepressant effect.
Go to : 
ACKNOWLEDGEMENTS
We thank Manipal University, Manipal and Farooqia College of Pharmacy, Mysore for providing the infrastructure and other facilties.
Go to : 
References
1. Kar K, Puri VN, Patnaik GK, Sur RN, Dhawan BN, Kulshrestha DK, Rastogi RP. Spasmolytic constituents of Cedrus deodara (Roxb.) Loud: pharmacological evaluation of himachalol. J Pharm Sci. 1975; 64:258–262. PMID: 47907.

2. Shinde U, Phadke A, Nair A, Mungantiwar A, Dikshit V, Saraf M. Preliminary studies on the immunomodulatory activity of Cedrus deodara wood oil. Fitoterapia. 1999; 70:333–339.

3. Saxena A, Saxena AK, Singh J, Bhushan S. Natural antioxidants synergistically enhance the anticancer potential of AP9-cd, a novel lignan composition from Cedrus deodara in human leukemia HL-60 cells. Chem Biol Interact. 2010; 188:580–590. PMID: 20932957.

4. Dikshit A, Dixit S. Cedrus oil--a promising antifungal agent. Indian Perfumer. 1982; 26:216–227.
5. Bisht LS, Brindavanam NB, Kimothic P. Comparative study of herbal agents used for fumigtation in relations to formulation(*). Anc Sci Life. 1988; 8:125–132. PMID: 22557643.
6. Phillipson JD, Anderson LA. Ethnopharmacology and Western medicine. J Ethnopharmacol. 1989; 25:61–72. PMID: 2654491.

7. Gupta PP, Kulshrestha DK, Patnaik GK. Antiallergic activity of cedrus deodara. J Med Aromatic Plant Sci. 1997; 19:1007–1008.
8. Tiwari AK, Srinivas PV, Kumar SP, Rao JM. Free radical scavenging active components from Cedrus deodara. J Agric Food Chem. 2001; 49:4642–4645. PMID: 11600001.

9. Nisha M, Kalyanasundaram M, Paily KP, Abidha , Vanamail P, Balaraman K. In vitro screening of medicinal plant extracts for macrofilaricidal activity. Parasitol Res. 2007; 100:575–579. PMID: 17013649.

10. Singh A, Singh DK. Effect of herbal molluscicides and their combinations on the reproduction of the snail Lymnaea acuminata. Arch Environ Contam Toxicol. 2004; 46:470–477. PMID: 15253044.

11. Shivanand P, Viral D, Manish G, Subhash V, Jaganathan K. Formulation and evaluation of cedrus deodara loud extract. Int J Chem Tech Res. 2009; 100:1145–1152.
12. Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol. 2007; 110:200–234. PMID: 17276637.

13. Ramesh C, Krishnadas N, Radhakrishnan R, Rangappa S, Viswanatha GLS, Rajesh D, Gopal M, Talwar S. Anti-urolithiatic activity of heart wood extract of cedrus deodara in rats. J Complement Integr Med. 2010; 7:1–9.

14. Kumar A, Singh V, Chaudhary AK. Gastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud. in Wistar rats. J Ethnopharmacol. 2011; 134:294–297. PMID: 21182918.

15. Dhayabaran D, Florance EJ, Nandakumar K, Shanmugarathinam A, Puratchikody A. Anticonvulsant activity of fraction isolated from ethanolic extract of heartwood of Cedrus deodara. J Nat Med. 2014; 68:310–315. PMID: 23959538.

16. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985; 85:367–370. PMID: 3923523.

17. Lee JK. Anti-depressant like effect of methyl gallate isolated from acer barbinerve in mice. Korean J Physiol Pharmacol. 2013; 17:441–446. PMID: 24227946.
18. Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005; 177:245–255. PMID: 15609067.

19. Lakshmana MK, Raju TR. An isocratic assay for norepinephrine, dopamine, and 5-hydroxytryptamine using their native fluorescence by high-performance liquid chromatography with fluorescence detection in discrete brain areas of rat. Anal Biochem. 1997; 246:166–170. PMID: 9073352.

20. Akiskal HS, Van Valkenburg C. Mood disorders. Diagnostic Interviewing. Springer;1994. p. 79–107.
21. Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007; 12:331–359. PMID: 17389902.

22. Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007; 24:495–517. PMID: 17117412.

23. Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000; 47:305–313. PMID: 10686265.

24. Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974; 36:248–257. PMID: 4829619.

25. Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011; 7:121–147. PMID: 21225412.

26. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effect of Berberine on Depression- and Anxiety-Like Behaviors and Activation of the Noradrenergic System Induced by Development of Morphine Dependence in Rats. Korean J Physiol Pharmacol. 2012; 16:379–386. PMID: 23269899.

27. Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005; 177:245–255. PMID: 15609067.

28. Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004; 9:326–357. PMID: 14743184.

29. Zhou Q, Liu M. Doubt about antidepressant-like effect. J Biomed Res. 2013; 27:245–248. PMID: 23720683.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download